EFA6, exchange factor for ARF6, regulates the actin cytoskeleton and associated tight junction in response to E-cadherin engagement - PubMed (original) (raw)
EFA6, exchange factor for ARF6, regulates the actin cytoskeleton and associated tight junction in response to E-cadherin engagement
Frédéric Luton et al. Mol Biol Cell. 2004 Mar.
Abstract
We addressed the role of EFA6, exchange factor for ARF6, during the development of epithelial cell polarity in Madin-Darby canine kidney cells. EFA6 is located primarily at the apical pole of polarized cells, including the plasma membrane. After calcium-triggered E-cadherin-mediated cell adhesion, EFA6 is recruited to a Triton X-100-insoluble fraction and its protein level is increased concomitantly to the accelerated formation of a functional tight junction (TJ). The expression of EFA6 results in the selective retention at the cell surface of the TJ protein occludin. This effect is due to EFA6 capacities to promote selectively the stability of the apical actin ring onto which the TJ is anchored, resulting in the exclusion of TJ proteins from endocytosis. Finally, our data suggest that EFA6 effects are achieved by the coordinate action of both its exchange activity and its actin remodeling C-terminal domain. We conclude that EFA6 is a signaling molecule that responds to E-cadherin engagement and is involved in TJ formation and stability.
Figures
Figure 1.
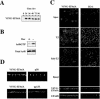
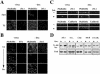
Biochemical and morphological analyses of MDCK cells expressing EFA6A. (A) Kinetic analysis by immunoblot of VSVG-EFA6A expression in MDCK cells after Dox removal. (B) Exogenously expressed VSVG-EFA6A activates the endogenous Arf6 protein. The amounts of GTP-bound endogenous Arf6 from lysates of MDCK-VSVG-EFA6A cells grown with or without Dox (48 h) were determined in a pull-down assay by using GST-GGA3(1-226) (top). Bottom, aliquots of total lysates. (C) Confocal IF analysis of VSVG-EFA6A and ZO-1 localization in MDCK cells expressing VSVG-EFA6A grown in the absence of Dox for 48 h: four xy sections from the apex of the cells to the basal face are shown from top to bottom, and an xz section is presented below. The xz section at the very bottom illustrates the labeling of endogenous EFA6B and ZO-1 in parental MDCK cells. (D) Xz sections of confocal IF images depicting the staining of the basolateral p58 and apical gp135 markers compared with VSVG-EFA6A.
Figure 2.
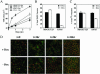
EFA6A expression accelerates the formation of a functional TJ. Cells were submitted to calcium starvation for 6 h followed by the addition of calcium. The indicated cell monolayers were analyzed for their TER (A), 9-kDa-dextran RITC paracellular diffusion (B), [14C]mannitol paracellular diffusion (C). (D) Confocal IF analysis at the TJ of MDCK-VSVG-EFA6A cells grown in the presence or absence of Dox (48 h). Cells were stained for occludin (green) and ZO-1 (red).
Figure 3.
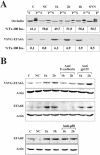
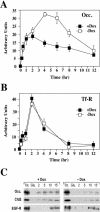
EFA6 responds to E-cadherin engagement before TJ formation. (A) VSVG-EFA6A and occludin Triton X-100 partitioning during calcium switch analyzed by immunoblots. The percentages of the Triton X-100–insoluble fraction of occludin and VSVG-EFA6A measured by densitometric analysis are indicated below the immunoblots. (B) Immunoblot analysis of the level of expression of VSVG-EFA6A detected with the anti-VSVG–specific antibody, and of the endogenous EFA6B protein detected with a rabbit-specific antiserum, in response to E-cadherin engagement. The same amount of proteins was loaded in each lane and controlled by anti-actin immunoblot of the corresponding gels. C, control cells grown in normal calcium medium; NC, cells incubated in the absence of calcium for 6 h; 1 and 2 h, time after calcium addition after the 6-h calcium depletion period. When indicated, the antibodies were added with the calcium.
Figure 4.
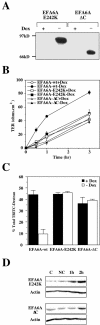
Exchange activity and the C-terminal actin remodeling domains of EFA6A are required to accelerate TJ formation. (A) Expression of VSVG-EFA6A-E242K and VSVG-EFA6A-ΔC in MDCK cells were analyzed by immunoblot by using the anti-VSVG antibody. Cells were submitted to a calcium switch and were analyzed for TER (B) and 9-kDa-dextran RITC paracellular diffusion (C). (D) Immunoblot analysis of the level of expression of the mutated proteins detected with the anti-VSVG–specific antibody in response to E-cadherin engagement. The same amount of proteins was loaded in each lane and controlled by anti-actin immunoblot of the corresponding gels. C, control cells grown in normal calcium medium; NC, cells incubated in the absence of calcium for 6 h; 1 and 2 h, time after calcium addition after the 6-h calcium depletion period.
Figure 5.
EFA6A delays the disassembly of TJ. The indicated cell monolayers were analyzed for their TER (A) and (RITC)-dextran 9-kDa paracellular diffusion (B) during TJ disassembly upon calcium removal.
Figure 6.
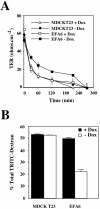
EFA6A promotes the cell surface retention of TJ proteins. The quantity of occludin (A) and Tf-R (B) present at the PM of MDCK-VSVG-EFA6A cells grown in the presence or absence of Dox (48 h) were determined by pulse-chase and cell-surface biotinylation (for details, see MATERIALS AND METHODS). The amounts of protein were quantified by densitometry and plotted against time. (C) Biotinylation endocytosis assay of MDCK-VSVG-EFA6A cells grown in the presence or absence of Dox (48 h) for occludin, claudin 2, and the EGF-R (for details, see MATERIALS AND METHODS). Immunoblots for the total amount of biotinylated protein (Tot.), the control for glutathione stripping after biotinylation (Glu.), the protected amount of proteins from glutathione stripping after 2-, 5-, 10-, and 15-min internalization were quantified by densitometry and plotted against time.
Figure 7.
EFA6A accelerates the polarized reorganization of the actin cytoskeleton and anchorage of the TJ proteins. (A) Confocal analysis of actin and ZO-1 during a calcium switch of MDCK-VSVG-EFA6A cells grown in the presence or absence of Dox (48 h). Single xy sections taken at the level of the cell-cell contacts and at the apex of the cells are shown 3 h after calcium addition. (B) Confocal analysis of actin and ZO-1 in polarized MDCK-VSVG-EFA6A cells grown in the presence or absence of Dox (48 h) after a 15-min treatment with latrunculin B (5 μM). Single xy sections taken at the TJ, sub-TJ, and nuclei levels are shown. Two independent xz sections are presented at the bottom. (C) Xz sections of confocal IF analysis of actin and ZO-1 (top) or actin and E-cadherin (bottom) in polarized MDCK-VSVG-EFA6A cells grown (+/- Dox 48 h) after or not a 15-min treatment with latrunculin B (5 μM) (Lat. +/-). (D) Immunoblot analysis of the total amount or the Triton X-100–insoluble fraction of ZO-1, occludin, claudin 2, Tf-R, and E-cadherin in polarized MDCK-VSVG-EFA6A cells grown in the presence or absence of Dox.
Similar articles
- EFA6 facilitates the assembly of the tight junction by coordinating an Arf6-dependent and -independent pathway.
Klein S, Partisani M, Franco M, Luton F. Klein S, et al. J Biol Chem. 2008 Oct 31;283(44):30129-38. doi: 10.1074/jbc.M803375200. Epub 2008 Sep 8. J Biol Chem. 2008. PMID: 18779331 Free PMC article. - The pleckstrin homology domain of the Arf6-specific exchange factor EFA6 localizes to the plasma membrane by interacting with phosphatidylinositol 4,5-bisphosphate and F-actin.
Macia E, Partisani M, Favard C, Mortier E, Zimmermann P, Carlier MF, Gounon P, Luton F, Franco M. Macia E, et al. J Biol Chem. 2008 Jul 11;283(28):19836-44. doi: 10.1074/jbc.M800781200. Epub 2008 May 19. J Biol Chem. 2008. PMID: 18490450 - The EFA6 family: guanine nucleotide exchange factors for ADP ribosylation factor 6 at neuronal synapses.
Sakagami H. Sakagami H. Tohoku J Exp Med. 2008 Mar;214(3):191-8. doi: 10.1620/tjem.214.191. Tohoku J Exp Med. 2008. PMID: 18323689 Review. - EFA6 in Axon Regeneration, as a Microtubule Regulator and as a Guanine Nucleotide Exchange Factor.
Gonzalez G, Chen L. Gonzalez G, et al. Cells. 2021 May 26;10(6):1325. doi: 10.3390/cells10061325. Cells. 2021. PMID: 34073530 Free PMC article. Retracted. Review.
Cited by
- EFA6B regulates a stop signal for collective invasion in breast cancer.
Fayad R, Rojas MV, Partisani M, Finetti P, Dib S, Abelanet S, Virolle V, Farina A, Cabaud O, Lopez M, Birnbaum D, Bertucci F, Franco M, Luton F. Fayad R, et al. Nat Commun. 2021 Apr 13;12(1):2198. doi: 10.1038/s41467-021-22522-4. Nat Commun. 2021. PMID: 33850160 Free PMC article. - The N and C termini of ZO-1 are surrounded by distinct proteins and functional protein networks.
Van Itallie CM, Aponte A, Tietgens AJ, Gucek M, Fredriksson K, Anderson JM. Van Itallie CM, et al. J Biol Chem. 2013 May 10;288(19):13775-88. doi: 10.1074/jbc.M113.466193. Epub 2013 Apr 3. J Biol Chem. 2013. PMID: 23553632 Free PMC article. - Proteomic and bioinformatic analysis of epithelial tight junction reveals an unexpected cluster of synaptic molecules.
Tang VW. Tang VW. Biol Direct. 2006 Dec 8;1:37. doi: 10.1186/1745-6150-1-37. Biol Direct. 2006. PMID: 17156438 Free PMC article. - USP9x-mediated deubiquitination of EFA6 regulates de novo tight junction assembly.
Théard D, Labarrade F, Partisani M, Milanini J, Sakagami H, Fon EA, Wood SA, Franco M, Luton F. Théard D, et al. EMBO J. 2010 May 5;29(9):1499-509. doi: 10.1038/emboj.2010.46. Epub 2010 Mar 25. EMBO J. 2010. PMID: 20339350 Free PMC article. - The C-terminal domain of EFA6A interacts directly with F-actin and assembles F-actin bundles.
Macia E, Partisani M, Wang H, Lacas-Gervais S, Le Clainche C, Luton F, Franco M. Macia E, et al. Sci Rep. 2019 Dec 16;9(1):19209. doi: 10.1038/s41598-019-55630-9. Sci Rep. 2019. PMID: 31844082 Free PMC article.
References
- Balda, M.S., and Matter, K. (1998). Tight junctions. J. Cell Sci. 111, 541-547. - PubMed
- Beck, K.A., and Nelson, W.J. (1996). The spectrin-based membrane skeleton as a membrane protein-sorting machinery. Am. J. Physiol. 270, C1263-C1270. - PubMed
- Cereijido, M., Shoshani, L., and Contreras, R.G. (2000). Biogenesis of tight junctions and epithelial polarity. Am. J. Physiol. 279, G477-G482. - PubMed
- Cereijido, M., Valdés, J., Shoshani, L., and Contreras, R.G. (1998). Role of tight junctions in establishing and maintaining cell polarity. Annu. Rev. Physiol. 60, 161-177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous