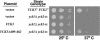The yeast casein kinase Yck3p is palmitoylated, then sorted to the vacuolar membrane with AP-3-dependent recognition of a YXXPhi adaptin sorting signal - PubMed (original) (raw)
The yeast casein kinase Yck3p is palmitoylated, then sorted to the vacuolar membrane with AP-3-dependent recognition of a YXXPhi adaptin sorting signal
Beimeng Sun et al. Mol Biol Cell. 2004 Mar.
Abstract
Our previous work found the two yeast plasma membrane-localized casein kinases Yck1p and Yck2p to be palmitoylated on C-terminal Cys-Cys sequences by the palmitoyl transferase Akr1p. The present work examines a third casein kinase, Yck3p, which ends with the C-terminal sequence Cys-Cys-Cys-Cys-Phe-Cys-Cys-Cys. Yck3p is palmitoylated and localized to the vacuolar membrane. While the C-terminal cysteines are required for this palmitoylation, Akr1p is not. Palmitoylation requires the C-terminal Yck3p residues 463-524, whereas information for vacuolar sorting maps to the 409-462 interval. Vacuolar sorting is disrupted in cis through deletion of the 409-462 sequences and in trans through mutation of the AP-3 adaptin complex; both cis- and trans-mutations result in Yck3p missorting to the plasma membrane. This missorted Yck3p restores 37 degrees C viability to yck1Delta yck2-ts cells. yck1Delta yck2-ts suppressor mutations isolated within the YCK3 gene identify the Yck3p vacuolar sorting signal-the tetrapeptide YDSI, a perfect fit to the YXXPhi adaptin-binding consensus. Although YXXPhi signals have a well-appreciated role in the adaptin-mediated sorting of mammalian cells, this is the first signal of this class to be identified in yeast.
Figures
Figure 5.
Yck3p traffics to the vacuole via the AP-3 pathway. HA-tagged Yck3p (A) or HA-tagged Yck232 (B) expressed at normal endogenous levels under the respective control of YCK3 or YCK2 upstream sequences, in either the wild-type Saccharomyces Deletion Consortium strain or in the isogenic consortium strains individually deleted for the indicated genes. For each mutant strain, two typical cells are shown, with the fluorescent images of the cells on the left and the same cells visualized by DIC optics shown to the right.
Figure 1.
Yck3p is subject to Akr1p-independent palmitoylation. Yck2 and Yck3 proteins, tagged at their amino termini with a 6xHis/FLAG/HA sequence, were expressed from the GAL1 promoter in either wild-type (AKR1+) cells or isogenic _akr1_Δ cells. The ΔCys mutation is a deletion of the last C-terminal eight Yck3p residues, CCCCFCCC. After labeling of the cells with [3H]palmitic acid, extracts were prepared, the tagged kinases were immune precipitated and subjected to SDS-PAGE and fluorography (top). Recovery of the immune-precipitated kinases was assessed by Western blotting by using an anti-HA monoclonal antibody (bottom).
Figure 2.
Yck3p localizes to the vacuolar membrane. Indirect immunofluorescent detection of Yck3 proteins is through amino-terminal HA epitope tags. The tagged constructs were expressed at normal endogenous levels under the control of YCK3 upstream sequences or in one instance, overexpressed from the GAL1 promoter (GAL1 _P_-Yck3p). Two typical cells for each condition are shown, with the fluorescent images of the cells on the left and the same cells as visualized by DIC optics shown to the right.
Figure 3.
Deletional mapping of the Yck3 sequences that direct palmitoylation and vacuolar localization. (A) Schematic of Ura3-Yck3 fusion proteins. At the top is a schematic of Yck3p; the highly conserved (among the Yck proteins) N-terminal kinase domain is followed by the poorly conserved C-terminal domain, terminating in the indicated cysteine-rich sequence. The different Ura3-Yck3 fusion protein constructs with the indicated portions of Yck3 fused in-frame to the C terminus of the cytoplasmic enzyme Ura3p are shown below. The Ura3 amino terminus is tagged with a copy of the HA epitope to facilitate immune detection. (B) Indirect immunofluorescent localization of the Ura3-Yck3 constructs. Fusion proteins were expressed from the GAL1 P. For each construct, two typical cells are shown, with the fluorescent images of the cells on the left and the same cells visualized by DIC optics shown to the right.
Figure 4.
The Yck3 409-462 sequence interval harbors a vacuolar sorting signal. (A) Schematic of the different HA-tagged Yck mutant constructs. (B) Indirect immunofluorescent localization of the different HA-tagged mutant Yck proteins. Expression of the Yck2 and Yck3 constructs was directed by the natural YCK2 and YCK3 upstream regulatory sequences. Two typical cells are shown for each construct with the fluorescent images of the cells on the left and the same cells visualized by DIC optics shown to the right.
Figure 6.
Yck3p missorting confers 37°C viability to _yck1_Δ yck2-ts cells. Serial 10-fold dilutions of _yck1_Δ yck2-ts cells, transformed by the CEN/ARS vector pRS316 or by pRS316 carrying either the wild-type YCK3 or the mutant _YCK3_Δ409-462 allele, were plated at both permissive (25°C) and nonpermissive (37°C) temperatures. Growth of the _yck1_Δ yck2-ts transformants is compared with that of the isogenic YCK1+ YCK2+ parental strain at the two temperatures.
Figure 7.
Point mutations within the Yck3 YDSI tetrapeptide disrupts vacuolar sorting. (A) Mutations within the Yck3 YDSI tetrapeptide conferring _yck1_Δ yck2-ts suppression. The ten residue-long Yck3p sequence harboring the YDSI tetrapeptide is shown. Just below are the substitutions identified from the seven mutants having only a single substitution within the 409-462 interval. Below this are substitutions within YDSI peptide found among the seven mutants that had more than one amino acid changes within the 409-462 interval. For substitutions that were reisolated multiple times, the number of times the particular change was found is indicated (in parentheses). (B-D) Effects of YDSI mutations on localization of HA-tagged Yck3 and Yck232 proteins. Localization of wild-type and mutant versions of Yck3p and the chimeric Yck232p, expressed under the control of YCK3 and YCK2 promoter sequences, respectively, were detected by indirect immunofluorescent microscopy. For each mutant, two typical cells are shown, with the fluorescent images of the cells on the left and the same cells visualized by DIC optics shown to the right. (B) Effects of the indicated suppressor mutations on Yck3p localization. (C) Effect of suppressor mutations within the Yck232 chimeric protein context. (D) Effect of alanine substitutions at each position within the YDSI tetrapeptide tested on Yck232p localization.
Similar articles
- Akr1p-dependent palmitoylation of Yck2p yeast casein kinase 1 is necessary and sufficient for plasma membrane targeting.
Babu P, Deschenes RJ, Robinson LC. Babu P, et al. J Biol Chem. 2004 Jun 25;279(26):27138-47. doi: 10.1074/jbc.M403071200. Epub 2004 Apr 22. J Biol Chem. 2004. PMID: 15105419 - Prenylated isoforms of yeast casein kinase I, including the novel Yck3p, suppress the gcs1 blockage of cell proliferation from stationary phase.
Wang X, Hoekstra MF, DeMaggio AJ, Dhillon N, Vancura A, Kuret J, Johnston GC, Singer RA. Wang X, et al. Mol Cell Biol. 1996 Oct;16(10):5375-85. doi: 10.1128/MCB.16.10.5375. Mol Cell Biol. 1996. PMID: 8816449 Free PMC article. - Akr1p and the type I casein kinases act prior to the ubiquitination step of yeast endocytosis: Akr1p is required for kinase localization to the plasma membrane.
Feng Y, Davis NG. Feng Y, et al. Mol Cell Biol. 2000 Jul;20(14):5350-9. doi: 10.1128/MCB.20.14.5350-5359.2000. Mol Cell Biol. 2000. PMID: 10866691 Free PMC article. - Receptor-mediated protein sorting to the vacuole in yeast: roles for a protein kinase, a lipid kinase and GTP-binding proteins.
Stack JH, Horazdovsky B, Emr SD. Stack JH, et al. Annu Rev Cell Dev Biol. 1995;11:1-33. doi: 10.1146/annurev.cb.11.110195.000245. Annu Rev Cell Dev Biol. 1995. PMID: 8689553 Review. - Compartmentalized transport, modification, and sorting of yeast vacuolar hydrolases.
Vida TA, Herman PK, Emr SD, Graham TR. Vida TA, et al. Biomed Biochim Acta. 1991;50(4-6):413-20. Biomed Biochim Acta. 1991. PMID: 1801705 Review. No abstract available.
Cited by
- Casein kinase 1γ ensures monopolar growth polarity under incomplete DNA replication downstream of Cds1 and calcineurin in fission yeast.
Koyano T, Konishi M, Martin SG, Ohya Y, Hirata D, Toda T, Kume K. Koyano T, et al. Mol Cell Biol. 2015 May;35(9):1533-42. doi: 10.1128/MCB.01465-14. Epub 2015 Feb 17. Mol Cell Biol. 2015. PMID: 25691662 Free PMC article. - A lysosomal biogenesis map reveals the cargo spectrum of yeast vacuolar protein targeting pathways.
Eising S, Esch B, Wälte M, Vargas Duarte P, Walter S, Ungermann C, Bohnert M, Fröhlich F. Eising S, et al. J Cell Biol. 2022 Apr 4;221(4):e202107148. doi: 10.1083/jcb.202107148. Epub 2022 Feb 17. J Cell Biol. 2022. PMID: 35175277 Free PMC article. - Yeast cell death pathway requiring AP-3 vesicle trafficking leads to vacuole/lysosome membrane permeabilization.
Stolp ZD, Kulkarni M, Liu Y, Zhu C, Jalisi A, Lin S, Casadevall A, Cunningham KW, Pineda FJ, Teng X, Hardwick JM. Stolp ZD, et al. Cell Rep. 2022 Apr 12;39(2):110647. doi: 10.1016/j.celrep.2022.110647. Cell Rep. 2022. PMID: 35417721 Free PMC article. - Seed-expressed casein kinase I acts as a positive regulator of the SeFAD2 promoter via phosphorylation of the SebHLH transcription factor.
Kim MJ, Go YS, Lee SB, Kim YS, Shin JS, Min MK, Hwang I, Suh MC. Kim MJ, et al. Plant Mol Biol. 2010 Jul;73(4-5):425-37. doi: 10.1007/s11103-010-9630-7. Epub 2010 Mar 28. Plant Mol Biol. 2010. PMID: 20349267 - The yeast kinase Yck2 has a tripartite palmitoylation signal.
Roth AF, Papanayotou I, Davis NG. Roth AF, et al. Mol Biol Cell. 2011 Aug 1;22(15):2702-15. doi: 10.1091/mbc.E11-02-0115. Epub 2011 Jun 8. Mol Biol Cell. 2011. PMID: 21653825 Free PMC article.
References
- Babu, P., Bryan, J.D., Panek, H.R., Jordan, S.L., Forbrich, B.M., Kelley, S.C., Colvin, R.T., and Robinson, L.C. (2002). Plasma membrane localization of the Yck2p yeast casein kinase 1 isoform requires the C-terminal extension and secretory pathway function. J. Cell Sci. 115, 4957-4968. - PubMed
- Boehm, M., and Bonifacino, J.S. (2002). Genetic analyses of adaptin function from yeast to mammals. Gene 286, 175-186. - PubMed
- Bonifacino, J.S., and Traub, L.M. (2003). Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72, 395-447. - PubMed
- Chen, L., and Davis, N.G. (2002). Ubiquitin-independent entry into the yeast recycling pathway. Traffic 3, 110-123. - PubMed
- Conibear, E., and Stevens, T.H. (1998). Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim. Biophys. Acta 1404, 211-230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases