A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2 - PubMed (original) (raw)
A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2
Swee Kee Wong et al. J Biol Chem. 2004.
Abstract
The coronavirus spike (S) protein mediates infection of receptor-expressing host cells and is a critical target for antiviral neutralizing antibodies. Angiotensin-converting enzyme 2 (ACE2) is a functional receptor for the coronavirus (severe acute respiratory syndrome (SARS)-CoV) that causes SARS. Here we demonstrate that a 193-amino acid fragment of the S protein (residues 318-510) bound ACE2 more efficiently than did the full S1 domain (residues 12-672). Smaller S protein fragments, expressing residues 327-510 or 318-490, did not detectably bind ACE2. A point mutation at aspartic acid 454 abolished association of the full S1 domain and of the 193-residue fragment with ACE2. The 193-residue fragment blocked S protein-mediated infection with an IC(50) of less than 10 nm, whereas the IC(50) of the S1 domain was approximately 50 nm. These data identify an independently folded receptor-binding domain of the SARS-CoV S protein.
Figures
Fig. 1
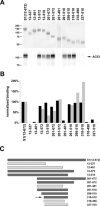
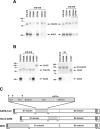
Residues 318–510 of the SARS-CoV S protein include the receptor-binding domain.A, S1-Ig, containing S protein residues 12–672 fused to the Fc region of human IgG1, or truncation variants of S1-Ig containing the indicated S protein residues, were purified from media of transfected 293T cells. S1-Ig and variants were normalized for expression, as shown by Coomassie staining (top panel), and used to precipitate soluble metabolically labeled ACE2 (bottom panel). Precipitates were analyzed by SDS-PAGE, and ACE2 was quantified by phosphorimaging. B, the indicated S1-Ig variants were incubated with ACE2-transfected 293T cells and analyzed by flow cytometry (black bars) or used, as in (A), to immunoprecipitate soluble ACE2 (gray bars). Bars indicate averages of two or more experiments normalized to results for S1-Ig. C, representation of truncation variants assayed in A and B. Dark gray indicates association with ACE2 greater than 25% of that observed for S1-Ig in both precipitation and flow-cytometry assays. Light gray indicates ACE2 association less than 10%, in both assays, of that for S1-Ig. The arrow indicates 318–510 variant, the smallest fragment observed to bind ACE2.
Fig. 2
An S1-Ig variant containing residues 318–510 associates with ACE2 and blocks S protein-mediated entry better than does S1-Ig.A, S1-Ig, or variants containing residues 318–510 or 12–327, were purified from transfected 293T cells and quantified. An aliquot of each variant diluted to the indicated concentrations was visualized by SDS-PAGE and Coomassie staining (top panel) and used to precipitate soluble metabolically labeled ACE2. Precipitates were analyzed by SDS-PAGE, and ACE2 was quantified by phosphorimaging. B, ACE2 precipitated by the indicated concentrations of S1-Ig and the indicated variants. An average of two experiments is shown. C, the indicated concentrations of S1-Ig, or of the 318–510 or 12–327 variants, were incubated with 293T cells expressing ACE2, together with an SIV modified to express green fluorescent protein (SIV-GFP) and pseudotyped with S protein of SARS-CoV or with VSV-G. Infection with pseudotyped virus was quantified by measuring green fluorescence by flow cytometry and shown here as mean fluorescent intensity (m.f.i.). D, fluorescent microscopic fields of 293T cells transfected with ACE2 and incubated with SIV-GFP pseudotyped with S protein in the presence of a 250 n
m
concentration of the 12–327 (right) or the 318–510 (left) variants. Many microscopic fields of cells incubated with the 318–510 variant lacked observable fluorescing cells.
Fig. 3
Analysis of point mutations of S1-Ig and the 318–510 variant.A, the 318–510 S1-Ig truncation variant, or variants thereof in which each of seven cysteines was altered individually to alanine, were analyzed as described in the legend to Fig. 1. Variants in which cysteine 366 or 419 was altered to alanine did not express and were not further analyzed. A variant containing alterations of both cysteines 323 and 378 was also analyzed (right panel). B, 318–510 variants (left panel) or S1-Ig variants (right panel) in which glutamic acid 452 or aspartic acids 454, 463, or 480 were altered individually to alanine were analyzed as in Fig. 1. C, representation of the S proteins of SARS-CoV, HCoV-229E, and MHV, aligned by their S2 domains. Dark gray indicates leader and transmembrane sequences. Light gray indicates receptor-binding domain. The receptor-binding domain of SARS-CoV is shown with _N_-glycosylation sites (small circles) and cysteines indicated. Residues that make a substantial contribution to ACE2 association (glutamic acid 452 and aspartic acid 454) are shown as white bars.
Similar articles
- Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus.
Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Li W, et al. Nature. 2003 Nov 27;426(6965):450-4. doi: 10.1038/nature02145. Nature. 2003. PMID: 14647384 Free PMC article. - Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2.
Li W, Zhang C, Sui J, Kuhn JH, Moore MJ, Luo S, Wong SK, Huang IC, Xu K, Vasilieva N, Murakami A, He Y, Marasco WA, Guan Y, Choe H, Farzan M. Li W, et al. EMBO J. 2005 Apr 20;24(8):1634-43. doi: 10.1038/sj.emboj.7600640. Epub 2005 Mar 24. EMBO J. 2005. PMID: 15791205 Free PMC article. - Structure of SARS coronavirus spike receptor-binding domain complexed with receptor.
Li F, Li W, Farzan M, Harrison SC. Li F, et al. Science. 2005 Sep 16;309(5742):1864-8. doi: 10.1126/science.1116480. Science. 2005. PMID: 16166518 - Insights from the association of SARS-CoV S-protein with its receptor, ACE2.
Li W, Choe H, Farzan M. Li W, et al. Adv Exp Med Biol. 2006;581:209-18. doi: 10.1007/978-0-387-33012-9_36. Adv Exp Med Biol. 2006. PMID: 17037532 Free PMC article. Review. No abstract available. - Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus.
Kuhn JH, Li W, Choe H, Farzan M. Kuhn JH, et al. Cell Mol Life Sci. 2004 Nov;61(21):2738-43. doi: 10.1007/s00018-004-4242-5. Cell Mol Life Sci. 2004. PMID: 15549175 Free PMC article. Review.
Cited by
- Cellular and Humoral Immune Responses in Covid-19 and Immunotherapeutic Approaches.
Hasan A, Al-Ozairi E, Al-Baqsumi Z, Ahmad R, Al-Mulla F. Hasan A, et al. Immunotargets Ther. 2021 Mar 9;10:63-85. doi: 10.2147/ITT.S280706. eCollection 2021. Immunotargets Ther. 2021. PMID: 33728277 Free PMC article. Review. - Molecular scaffolds from mother nature as possible lead compounds in drug design and discovery against coronaviruses: A landscape analysis of published literature and molecular docking studies.
Khursheed A, Jain V, Rasool A, Rather MA, Malik NA, Shalla AH. Khursheed A, et al. Microb Pathog. 2021 Aug;157:104933. doi: 10.1016/j.micpath.2021.104933. Epub 2021 May 11. Microb Pathog. 2021. PMID: 33984466 Free PMC article. Review. - What are the drugs having potential against COVID-19?
Kucukoglu K, Faydalı N, Bul D. Kucukoglu K, et al. Med Chem Res. 2020;29(11):1935-1955. doi: 10.1007/s00044-020-02625-1. Epub 2020 Sep 10. Med Chem Res. 2020. PMID: 32929317 Free PMC article. Review. - Structural and Functional Characterization of SARS-CoV-2 RBD Domains Produced in Mammalian Cells.
Gstöttner C, Zhang T, Resemann A, Ruben S, Pengelley S, Suckau D, Welsink T, Wuhrer M, Domínguez-Vega E. Gstöttner C, et al. Anal Chem. 2021 May 4;93(17):6839-6847. doi: 10.1021/acs.analchem.1c00893. Epub 2021 Apr 19. Anal Chem. 2021. PMID: 33871970 Free PMC article. - Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus.
Wu K, Peng G, Wilken M, Geraghty RJ, Li F. Wu K, et al. J Biol Chem. 2012 Mar 16;287(12):8904-11. doi: 10.1074/jbc.M111.325803. Epub 2012 Jan 30. J Biol Chem. 2012. PMID: 22291007 Free PMC article.
References
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. N. Engl. J. Med. 2003;348:1953–1966. - PubMed
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. N. Engl. J. Med. 2003;348:1967–1976. - PubMed
- Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W., Ling A.E., Chan P.K., Tam J.S., Zambon M.C., Gopal R., Drosten C., van der Werf S., Escriou N., Manuguerra J.C., Stohr K., Peiris J.S., Osterhaus A.D. Lancet. 2003;362:263–270. - PMC - PubMed
- Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. Science. 2003;300:1399–1404. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous