Knockout of ERK5 causes multiple defects in placental and embryonic development - PubMed (original) (raw)
Knockout of ERK5 causes multiple defects in placental and embryonic development
Lijun Yan et al. BMC Dev Biol. 2003.
Abstract
Background: ERK5 is a member of the mitogen activated protein kinase family activated by certain mitogenic or stressful stimuli in cells, but whose physiological role is largely unclear.
Results: To help determine the function of ERK5 we have used gene targeting to inactivate this gene in mice. Here we report that ERK5 knockout mice die at approximately E10.5. In situ hybridisation for ERK5, and its upstream activator MKK5, showed strong expression in the head and trunk of the embryo at this stage of development. Between E9.5 and E10.5, multiple developmental problems are seen in the ERK5-/- embryos, including an increase in apoptosis in the cephalic mesenchyme tissue, abnormalities in the hind gut, as well as problems in vascular remodelling, cardiac development and placental defects.
Conclusion: Erk5 is essential for early embryonic development, and is required for normal development of the vascular system and cell survival.
Figures
Figure 1
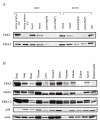
Generation of ERK5 knockout mice. A) ERK5 knockout mice were made using a targeting vector to delete exons 4 and 5 of the murine ERK5 gene through the addition of a neomycin selection cassette. A thymidine kinase cassette acts as a negative selection marker during ES cell selection. B) ES cell DNA was digested with both Hind III and Mfe I, and a Southern blot performed using a probe 3' to the targeting vector. The position of the wild type 9.5 kb fragment and targeted 3.3 kb fragment are indicated. C) DNA was isolated from E9.5 embryos and digested with Hind III and Mfe I. Southern blots were then probed with the 3' probe as described in (B)D) Soluble protein from homogenates of E9.5 embryos was run on 4–14% acrylamide gels. Immunoblotting was then carried out using antibodies which recognised ERK5, MKK5, ERK1/2 or p38.
Figure 2
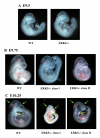
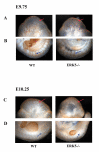
Phenotypes of ERK5 -/- mutant embryos. Embryos were isolated from timed matings of ERK5+/- mice and genotyped by PCR analysis of the isolated yolk sac. At E9.5 (A) little difference could be observed between wild type and ERK5-/- littermates. At E9.75 (B) differences could be seen between the WT and ERK5-/- embryos in the head regions, particularly in the cephalic mesenchyme of class II embryos (red arrowhead). At E10.25 (C) ERK5-/- embryos were growth retarded compared to wild type embryos. ERK5-/- embryos showed an abnormal head shape, compared to wild type embryos (green arrow) and in class II ERK5-/- embryos also showed severe abnormalities in the cephalic mesenchyme and 1st and 2nd branchial arches (yellow arrows). Development of the hind limb buds (star) and lower trunk was also retarded in the ERK5-/- embryos.
Figure 3
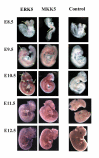
Expression of ERK5 and MKK5 during embryonic development. Whole mount in situ hybridisation was carried out on wild-type embryos as described in the methods using antisense RNA probes against ERK5 or MKK5 or with no RNA probe. Expression of ERK5 and MKK5 was analysed at E8.5 was seen in the cephalic neural fold and primitive gut. At E9.5 expression was also seen in the branchial arch, cephalic region and somites and lateral ridge of the body wall. From E10.5, E11.5 to E12.5 expression of ERK5 and MKK5 increases with high expression seen in the branchial arch, head and limb buds.
Figure 4
Analysis of ERK5 expression in embryo sections. Whole mount in situ hybridisation was carried out on wild-type embryos (A-C) or placentas (D) as described in the methods using antisense RNA probes against ERK5 at E9.5, E9.75 and E10.5. After staining embryos were sectioned on a vibrotone. Strong ERK5 expression was seen in the cephalic mesenchyme (star) branchial arch and neuroepithelium. Weak expression was seen in the heart at E9.5, however this increases by E10.25 (diamond). Strong ERK5 expression was seen in the sinus venous below the heart (arrow). In the placenta (D) strongest expression was seen in the chorionic plate and labyrinthine layers.
Figure 5
Immunoblotting of ERK5 and MKK5. Wild type embryos (E9.5 and 10.5) were dissected and head, heart, gut and placenta were isolated. The placenta was then subdivided into upper (mainly embryonic) and lower (mainly maternal) regions. Whole wild type and ERK5-/- embryos were also isolated at E9.5. Tissues were also isolated from adult wild type mice. Samples were homogenised, insoluble material removed by centrifugation, and the concentration of soluble protein in the extract determined by a Bradford assay. Soluble protein (30 μg) were run on 4–14% acrylamide gels. Immunoblotting was then carried out using antibodies which recognise ERK5 or MKK5 for both embryonic (A) and adult (B) samples. Levels of ERK1/2, p38 and actin were also determined in the adult tissue samples (B).
Figure 6
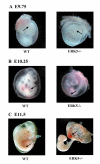
Morphology of wild type and ERK5 -/- mutant yolk sacs. Embryos were isolated from timed matings of ERK5+/- mice and photographed. Embryos were then genotyped by PCR analysis of the isolated yolk sac. At E9.75 (A) wild type and ERK5-/- mutant yolk contain blood islands (arrow). By E10.25 (B), the blood vessels found in wild type yolk sacs formed distinct large vessels which branched down into smaller vessels (arrow). In contrast, the surfaces of the ERK5-/- yolk sacs became pale, and did not show the branched blood vessels. At E11.5 (C), ERK5-/- yolk sacs appeared intermittent with diffuse patches of red blood cells (arrow). The ERK5-/- embryos showed were pale and apparently devoid of blood circulation without a beating heart.
Figure 7
CD31 whole-mount immunohistochemistry of embryos at E9.75 and E10.25. Embryos were isolated from timed mating and stained using an CD31 antibody as described in the Methods. At E9.75 (A) networks of large blood vessels were seen in the head regions of both wild type and ERK5-/- embryos (red arrow), and intersomitic vessels (blue arrow) were also apparent in both genotypes. By E10.25 however the blood vessels in the head region of wild type embryos had started to undergo angiogenesis to give rise to branched networks of smaller vessels. This was not seen in the ERK5-/- embryos (compare red arrows in B). Similarly more branching was seen in the intersomitic vessels in the wild type than ERK5-/- embryos at E10.25 (blue arrows). Results are representative of three independent experiments.
Figure 8
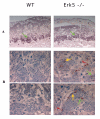
Histological sections of the heart at E9.75. E9.75 wild type and ERK5-/- embryos were isolated from timed matings and TS paraffin sections were taken and stained with haematoxylin and eosin as described in the methods. Normal patterning of the heart was observed in the ERK5-/- embryos, with both atrial (At) and ventrical (V) chambers. The thickness of the atrial wall (arrow) in ERK5-/- embryos was thinner that in wild type hearts (A). The average thickness of the atrium wall was quantified from 4 wild type and 4 ERK5-/- embryos (B). ERK5-/- embryos had a significant decrease in atrial wall thickness (P < 0.01)
Figure 9
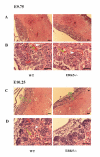
Histological sections of placenta at E9.75 and E10.25. Placentas were isolated from E9.75 and E10.25 wild type and ERK5-/- mice. Transverse paraffin sections were taken of the placentas and stained with haematoxylin and eosin as described. At E9.75, low (A) and high magnification (B) pictures of the TS sections are showed little difference between wild type and ERK5-/- embryos. At E9.75 both maternal (white arrowhead) and embryonic (green arrow) blood vessels, could be seen in both wild type and ERK5-/- placentas. At E10.25 low (C) and high magnification (D) showed that chorionic plate (CP), labyrinth (L) and spongiotrophoblast (S) layers are were present in both wild type and ERK5-/- embryos. At E10.25 intermixing of maternal and embryonic blood vessels (arrows) was seen, however in the ERK5-/- placentas many fewer maternal blood vessels were apparent in the labyrinthine layers. Scale bars are 0.1 mm and results are representative of three independent experiments.
Figure 10
4311 in situ and caspase 3 staining of E10.25 placentas. A) Wax sections of E10.25 wild type and ERK5-/- placentas were analysed by in situ hybridisation with an antisense RNA probe against 4311. The spongiotrophoblast layer is indicated by an arrow. B)Wild type and ERK5-/- placentas were isolated from E10.25 embryos, paraffin sections taken and then stained with an antibody which recognised cleaved caspase 3. Higher numbers of cleaved caspase 3 postitive cells were seen in ERK5-/- embryos compared to wild type controls. In the ERK5-/- placentas, apoptosis was seen in endothelial cells (red arrow), trophoblast cells (green arrow) and some embryonic blood cells (yellow arrow) within the labryinth. No apoptosis of giant cells was seen.
Figure 11
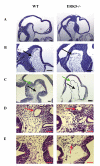
Analysis of cephalic mesenchyme. Wild type and ERK5-/- embryos were isolated from timed matings and LS or TS paraffin sections were taken and stained with haematoxylin and eosin as described in the methods. Analysis of LS sections at low (A) and high (B) magnification showed that there was less contact between the neuroepithelium and cephalic mesenchyme (black arrows) in ERK5-/- embryos, and that the cephalic mesenchyme was less dense with larger spaces between the cells in the ERK5-/- embryos (green arrows). Sections shown are representative of 5 wild type and 5 ERK5-/- embryos. LS sections were prepared from E10.25 embryos and stained with haematoxylin and eosin (C). The cephalic mesenchyme was almost completely absent in ERK5-/- embryos and the neuroepithelium was thinner. In ERK5-/- embryos at E9.75 the TS sections (D and E) through the cephalic mesenchyme again showed less mesenchymal tissue in the ERK5-/- embryos, however major blood vessels (arrows) were seen in both wild type and ERK5-/- embryos. In contrast to wild type embryos, where little bleeding was seen in the cephalic mesenchyme, in ERK5-/- embryos bleeding into the cephalic mesenchyme was frequently seen, especially where the mesenchymal tissue was absent (arrows in ERK5-/- embryos in D and E).
Figure 12
Analysis of apoptosis in the head of ERK5-/- embryos. The level of apoptosis in E9.75 embryos were analyses by whole mount TUNEL staining. These showed that there was greatly increased apoptosis in the ERK5-/- embryos compared to wild type littermate controls. (A) Fluorescent images of the head region of whole mount TUNEL stained embryos. (B) Light microscope pictures of the same regions at the same magnification. The cephalic mesenchyme is indicated by an arrowhead. Results are representative of 3 experiments.
Figure 13
Analysis of hind gut at E9.75. E9.75 wild type and ERK5-/- embryos were isolated from timed matings and TS paraffin sections were taken and stained with haematoxylin and eosin as described in the methods (A). TS sections through the guts of ERK5-/- mice showed several areas were there appeared to be hyperproliferation of the gut endothelium (arrow). This was not observed in wild type embryos. Similar results were seen in 3 wild type and 3 ERK5-/- embryos.
Similar articles
- Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects.
Regan CP, Li W, Boucher DM, Spatz S, Su MS, Kuida K. Regan CP, et al. Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9248-53. doi: 10.1073/pnas.142293999. Epub 2002 Jul 1. Proc Natl Acad Sci U S A. 2002. PMID: 12093914 Free PMC article. - Functions of MAP kinases: insights from gene-targeting studies.
Kuida K, Boucher DM. Kuida K, et al. J Biochem. 2004 Jun;135(6):653-6. doi: 10.1093/jb/mvh078. J Biochem. 2004. PMID: 15213239 Review. - Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development.
Adams RH, Porras A, Alonso G, Jones M, Vintersten K, Panelli S, Valladares A, Perez L, Klein R, Nebreda AR. Adams RH, et al. Mol Cell. 2000 Jul;6(1):109-16. Mol Cell. 2000. PMID: 10949032 - [Gene expression of extracellular-signal regulated protein kinase 5 and their MAPKK in fetal skin hypertrophic scars].
Chen W, Fu XB, Ge SL, Zhou G, Jiang DY, Sun TZ, Sheng ZY. Chen W, et al. Zhonghua Zheng Xing Wai Ke Za Zhi. 2004 May;20(3):222-4. Zhonghua Zheng Xing Wai Ke Za Zhi. 2004. PMID: 15449628 Chinese. - Regulation of cellular functions by the ERK5 signalling pathway.
Wang X, Tournier C. Wang X, et al. Cell Signal. 2006 Jun;18(6):753-60. doi: 10.1016/j.cellsig.2005.11.003. Epub 2006 Jan 6. Cell Signal. 2006. PMID: 16376520 Review.
Cited by
- ERK5 kinase activity is dispensable for cellular immune response and proliferation.
Lin EC, Amantea CM, Nomanbhoy TK, Weissig H, Ishiyama J, Hu Y, Sidique S, Li B, Kozarich JW, Rosenblum JS. Lin EC, et al. Proc Natl Acad Sci U S A. 2016 Oct 18;113(42):11865-11870. doi: 10.1073/pnas.1609019113. Epub 2016 Sep 27. Proc Natl Acad Sci U S A. 2016. PMID: 27679845 Free PMC article. - Laminar flow activation of ERK5 protein in vascular endothelium leads to atheroprotective effect via NF-E2-related factor 2 (Nrf2) activation.
Kim M, Kim S, Lim JH, Lee C, Choi HC, Woo CH. Kim M, et al. J Biol Chem. 2012 Nov 23;287(48):40722-31. doi: 10.1074/jbc.M112.381509. Epub 2012 Oct 5. J Biol Chem. 2012. PMID: 23043106 Free PMC article. - Extracellular signal-regulated kinase (ERK) 5 is necessary and sufficient to specify cortical neuronal fate.
Liu L, Cundiff P, Abel G, Wang Y, Faigle R, Sakagami H, Xu M, Xia Z. Liu L, et al. Proc Natl Acad Sci U S A. 2006 Jun 20;103(25):9697-702. doi: 10.1073/pnas.0603373103. Epub 2006 Jun 9. Proc Natl Acad Sci U S A. 2006. PMID: 16766652 Free PMC article. - Erk5 activation elicits a vasoprotective endothelial phenotype via induction of Kruppel-like factor 4 (KLF4).
Ohnesorge N, Viemann D, Schmidt N, Czymai T, Spiering D, Schmolke M, Ludwig S, Roth J, Goebeler M, Schmidt M. Ohnesorge N, et al. J Biol Chem. 2010 Aug 20;285(34):26199-210. doi: 10.1074/jbc.M110.103127. Epub 2010 Jun 15. J Biol Chem. 2010. PMID: 20551324 Free PMC article. - Role of the BMK1/ERK5 signaling pathway: lessons from knockout mice.
Hayashi M, Lee JD. Hayashi M, et al. J Mol Med (Berl). 2004 Dec;82(12):800-8. doi: 10.1007/s00109-004-0602-8. Epub 2004 Oct 28. J Mol Med (Berl). 2004. PMID: 15517128 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous