Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome - PubMed (original) (raw)
. 2003 Dec 23;100(26):15924-9.
doi: 10.1073/pnas.0306981100. Epub 2003 Dec 15.
Joji Yamamoto, Satoshi Iwasaki, Hiroshi Asaba, Hiroki Hamura, Yukio Ikeda, Mitsuhiro Watanabe, Kenta Magoori, Ryoichi X Ioka, Keisuke Tachibana, Yuichiro Watanabe, Yasutoshi Uchiyama, Koichi Sumi, Haruhisa Iguchi, Sadayoshi Ito, Takefumi Doi, Takao Hamakubo, Makoto Naito, Johan Auwerx, Masashi Yanagisawa, Tatsuhiko Kodama, Juro Sakai
Affiliations
- PMID: 14676330
- PMCID: PMC307669
- DOI: 10.1073/pnas.0306981100
Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome
Toshiya Tanaka et al. Proc Natl Acad Sci U S A. 2003.
Abstract
In this study, we defined the role of peroxisome proliferator-activated receptor beta/delta (PPARdelta) in metabolic homeostasis by using subtype selective agonists. Analysis of rat L6 myotubes treated with the PPARdelta subtype-selective agonist, GW501516, by the Affymetrix oligonucleotide microarrays revealed that PPARdelta controls fatty acid oxidation by regulating genes involved in fatty acid transport, beta-oxidation, and mitochondrial respiration. Similar PPARdelta-mediated gene activation was observed in the skeletal muscle of GW501516-treated mice. Accordingly, GW501516 treatment induced fatty acid beta-oxidation in L6 myotubes as well as in mouse skeletal muscles. Administration of GW501516 to mice fed a high-fat diet ameliorated diet-induced obesity and insulin resistance, an effect accompanied by enhanced metabolic rate and fatty acid beta-oxidation, proliferation of mitochondria, and a marked reduction of lipid droplets in skeletal muscles. Despite a modest body weight change relative to vehicle-treated mice, GW501516 treatment also markedly improved diabetes as revealed by the decrease in plasma glucose and blood insulin levels in genetically obese ob/ob mice. These data suggest that PPARdelta is pivotal to control the program for fatty acid oxidation in the skeletal muscle, thereby ameliorating obesity and insulin resistance through its activation in obese animals.
Figures
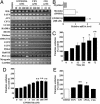
Fig. 1.
PPARδ agonists increase fatty acid oxidation in L6 myotubes. (A) RT-PCR analyses of PPARδ-regulated genes in L6 myotubes. (B) Real-time PCR analysis of PGC-1α mRNA levels in L6 myoblast/myotube cells exposed to GW501516. (C) Time responses on fatty acid oxidation in L6 myotubes exposed to GW501516. Cells were cultured with 100 nM GW501516 at the indicated times. (D) Dose responses on fatty acid oxidation in L6 myotubes exposed to GW501516. L6 myotubes were cultured with the indicated concentrations of GW501516 for 24 h. (E) The effect of the PPAR agonists on [14C]palmitate oxidation. After incubation in differentiation medium for 7 days, L6 myotubes were treated with DMSO (control), FEN (300 μM), GW501516 (100 nM), cPGI2 (10 μM), or CIG (30 μM) for 24 h. All assays were performed in triplicate, and each bar represents the mean ± SE of three to four independent experiments. *, P < 0.05; **, P < 0.01 compared with vehicle-treated control. HADHA, mitochondrial trifunctional protein; DECR, mitochondrial 2,4-dienoyl CoA reductase 1; HSL, hormone-sensitive lipase.
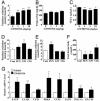
Fig. 2.
PPARδ agonist increases skeletal muscle fatty acid oxidation in C57BL/6J mice. Data in _A_-C show the effects of GW501516 on fatty acid oxidation in skeletal muscle (A), plasma TG (B), and NEFA (C). Data in _D_-F show the effect of PPAR agonists on fatty acid oxidation in skeletal muscle (D) and in liver (E), and serum total ketone body level (F). (G) Quantitative real-time PCR analyses in GW501516-treated skeletal muscle. GW501516 (3 mg/kg), FEN (300 mg/kg), or CIG (30 mg/kg) were orally administrated to mice for 7 days. Each bar represents the mean ± SE of four to five mice. *, P < 0.05; **, P < 0.01; difference from control, respectively.
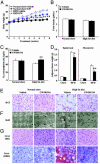
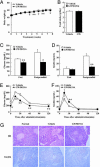
Fig. 3.
PPARδ agonist increases metabolic rate and attenuates body weight gain and adiposity in HFD-fed C57BL/6J mice. (A) Body weight change. (B) Food intake. (C) Oxygen consumption measurement. (D) Adipose tissue weight. (E) Morphological characterization of BAT (×40 magnification). (F) Electron microscopic analysis of mitochondrial biogenesis and lipid accumulation in skeletal muscle (×2,500 magnification). Asterisks, lipid droplets; arrows, mitochondria. (G) Morphological characterization of liver (×20 magnification). Each symbol or bar represents the mean ± SE of five mice. *, P < 0.05; **, P < 0.01; compared with vehicle-treated control. ORO, Oil red O.
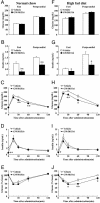
Fig. 4.
PPARδ agonist ameliorates insulin resistance in HFD-fed mice. (A and F) Plasma glucose. (B and G) Plasma insulin. (C and H) Plasma glucose levels during GTT. (D and I) Plasma insulin levels during GTT. (E and J) ITT. Each bar and symbol represents the mean ± SE of five mice. *, P < 0.05; **, P < 0.01; compared with vehicle-treated control.
Fig. 5.
PPARδ agonist improves insulin resistance and pancreatic islet hyperplasia in leptin-deficient ob/ob mice. (A) Body weight gain. (B) Food intake. (C) Plasma glucose. (D) Plasma insulin. (E and F) Plasma glucose and insulin levels during GTT. (G) Morphological changes in pancreatic islet in GW501516-treated ob/ob mice (×20 magnification). Each symbol or bar represents the mean ± SE of five mice. *, P < 0.05; **, P < 0.01; compared with vehicle-treated control.
Similar articles
- The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells.
Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. Dressel U, et al. Mol Endocrinol. 2003 Dec;17(12):2477-93. doi: 10.1210/me.2003-0151. Epub 2003 Oct 2. Mol Endocrinol. 2003. PMID: 14525954 - Activation of peroxisome proliferator-activated receptor-{delta} by GW501516 prevents fatty acid-induced nuclear factor-{kappa}B activation and insulin resistance in skeletal muscle cells.
Coll T, Alvarez-Guardia D, Barroso E, Gómez-Foix AM, Palomer X, Laguna JC, Vázquez-Carrera M. Coll T, et al. Endocrinology. 2010 Apr;151(4):1560-9. doi: 10.1210/en.2009-1211. Epub 2010 Feb 25. Endocrinology. 2010. PMID: 20185762 - The PPARdelta agonist, GW501516, promotes fatty acid oxidation but has no direct effect on glucose utilisation or insulin sensitivity in rat L6 skeletal muscle cells.
Dimopoulos N, Watson M, Green C, Hundal HS. Dimopoulos N, et al. FEBS Lett. 2007 Oct 2;581(24):4743-8. doi: 10.1016/j.febslet.2007.08.072. Epub 2007 Sep 6. FEBS Lett. 2007. PMID: 17869249 - Roles of peroxisome proliferator-activated receptor delta (PPARdelta) in the control of fatty acid catabolism. A new target for the treatment of metabolic syndrome.
Luquet S, Lopez-Soriano J, Holst D, Gaudel C, Jehl-Pietri C, Fredenrich A, Grimaldi PA. Luquet S, et al. Biochimie. 2004 Nov;86(11):833-7. doi: 10.1016/j.biochi.2004.09.024. Biochimie. 2004. PMID: 15589693 Review.
Cited by
- Activation of PPAR-δ induces microRNA-100 and decreases the uptake of very low-density lipoprotein in endothelial cells.
Fang X, Fang L, Liu A, Wang X, Zhao B, Wang N. Fang X, et al. Br J Pharmacol. 2015 Aug;172(15):3728-36. doi: 10.1111/bph.13160. Epub 2015 Jun 26. Br J Pharmacol. 2015. PMID: 25857370 Free PMC article. - IL-15 overexpression promotes endurance, oxidative energy metabolism, and muscle PPARδ, SIRT1, PGC-1α, and PGC-1β expression in male mice.
Quinn LS, Anderson BG, Conner JD, Wolden-Hanson T. Quinn LS, et al. Endocrinology. 2013 Jan;154(1):232-45. doi: 10.1210/en.2012-1773. Epub 2012 Nov 16. Endocrinology. 2013. PMID: 23161867 Free PMC article. - Transcriptional repression of mitochondrial function in aging: a novel role for the silencing mediator of retinoid and thyroid hormone receptors co-repressor.
Liu S, Reilly SM, Lee CH. Liu S, et al. Antioxid Redox Signal. 2013 Jul 20;19(3):299-309. doi: 10.1089/ars.2011.4413. Epub 2012 Aug 2. Antioxid Redox Signal. 2013. PMID: 22703297 Free PMC article. Review. - PPARs in the Control of Uncoupling Proteins Gene Expression.
Villarroya F, Iglesias R, Giralt M. Villarroya F, et al. PPAR Res. 2007;2007:74364. doi: 10.1155/2007/74364. PPAR Res. 2007. PMID: 17389766 Free PMC article. - The prostacyclin analog beraprost sodium ameliorates characteristics of metabolic syndrome in obese Zucker (fatty) rats.
Sato N, Kaneko M, Tamura M, Kurumatani H. Sato N, et al. Diabetes. 2010 Apr;59(4):1092-100. doi: 10.2337/db09-1432. Epub 2010 Jan 12. Diabetes. 2010. PMID: 20068136 Free PMC article.
References
- Spiegelman, B. M. & Flier, J. S. (2001) Cell 104, 531-543. - PubMed
- Taylor, S. I. (1999) Cell 97, 9-12. - PubMed
- Zimmet, P., Alberti, K. G. & Shaw, J. (2001) Nature 414, 782-787. - PubMed
- Willson, T. M., Brown, P. J., Sternbach, D. D. & Henke, B. R. (2000) J. Med. Chem. 43, 527-550. - PubMed
- Braissant, O., Foufelle, F., Scotto, C., Dauca, M. & Wahli, W. (1996) Endocrinology 137, 354-366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous