Beta subunit phosphorylation selectively increases fast desensitization and prolongs deactivation of alpha1beta1gamma2L and alpha1beta3gamma2L GABA(A) receptor currents - PubMed (original) (raw)
Beta subunit phosphorylation selectively increases fast desensitization and prolongs deactivation of alpha1beta1gamma2L and alpha1beta3gamma2L GABA(A) receptor currents
David J Hinkle et al. J Neurosci. 2003.
Abstract
We studied the effects of phosphorylation by protein kinase A (PKA) on GABA(A) receptors (alpha1beta1gamma2L andalpha1beta3gamma2L) transiently expressed in HEK 293T cells. Under conditions favorable for PKA activation, currents obtained using whole-cell patch clamp of lifted cells displayed increased rate and extent of the fast phases of desensitization, decreased rate of current deactivation after GABA removal, and prolongation of brief IPSC-like currents. Mutation of serine residues (beta1 S409, beta3 S407, beta3 S408) revealed that only beta1 S409 and beta3 S408 were critical for the modulatory effect of PKA on GABA(A) receptor currents. Additionally, repeated pulse inhibition was increased in receptors after mutation of the critical serine to glutamate and decreased when the serine was mutated to alanine. These data demonstrate that PKA phosphorylation modulated GABA(A) receptor currents by increasing fast phases of macroscopic desensitization and suggest a role for PKA in regulating GABAergic IPSC duration.
Figures
Figure 9.
Simulated currents approximated experimentally observed changes in current time course when rate of entry into desensitization was increased. A, Wild-type current time courses were simulated using the Haas and Macdonald (1999) model. B, The time constants listed here were corrected from the original publication that had a typographical error in which the rate constants (a1c, a2c, a3c, b1c, b2c, and b3c) for entry into distal closed states were transposed with the opening (a1o, a2o, a3o, b1o, b2o, and b3o) rates from the distal closed states. Units for all rate constants were (s-1), except for _k_off (M-1 s-1). Currents with decreased rate and extent of rapid macroscopic desensitization and accelerated macroscopic deactivation observed under conditions unfavorable to receptor phosphorylation were simulated by increasing both fast (_d_f) and intermediate (_d_i) entry rates into microscopic desensitized states in by 50% or by increasing the unbinding rate (_k_off) by 5000%. No significant change was seen when the entry rate (_d_s) into the slowest microscopic desensitization state (_D_s) was increased or decreased. (C, D) Decreasing _d_f and _d_i resulted in a simulated current time course qualitatively identical to experimental data for 6 sec, 400 msec, and 5 msec application of agonist, as did increasing _k_off (data not shown). E, Changing both _k_off and _d_f and _d_i resulted in simulated data with faster deactivation with 5 msec agonist applications. However, the reduction seen with increased _k_off was much greater than observed experimentally, whereas decreasing _d_f and _d_i by 50% approximated the prolongation in brief IPSC-like current well. F, When _d_f and _d_i were decreased 50%, the degree of repeated pulse inhibition was similar to that observed under dephosphorylating experimental conditions. A dashed line shows the peak current level of the second simulated agonist pulse using the unaltered model (w.t.) to aid comparison with the other simulations. Increasing koff resulted in simulated currents with a nearly complete loss of repeated pulse depression that was not observed experimentally. w.t., Wild type.
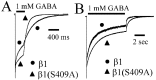
Figure 1.
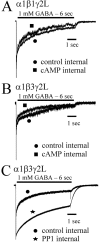
PKA modulation of α1β1γ2L and α1β3γ2L currents. GABA (1 m
m
) was rapidly applied for 6 sec to lifted HEK293T cells expressing α1β1γ2L (A) or α1β3γ2L (B, C) receptors. Data shown in all panels were normalized to peak and show GABA applications initiated 5 min after rupturing the patch and forming a whole-cell recording. Cells were then lifted from the surface of the dish and placed in the flow of bath solution from the multibarreled array of the rapid application system. Computer-initiated steps from the bath solution to the GABA solution elicited the recorded current. In separate populations of transfected cells, cAMP (300 μ
m
) was included in the intracellular recording pipette along with the standard intracellular solution used in controls (A, B). cAMP caused a modest increase in the rate and extent of desensitization. C, When PP1 was included in the intracellular pipette, the rate and extent of desensitization were decreased.
Figure 2.
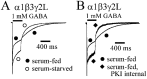
Modulation of α1β3γ2L currents by culture conditions and PKI. GABA (1 m
m
) was applied for 400 msec to lifted HEK293T cells expressing α1β3γ2L receptors. Serum-fed cells were cultured under standard cell conditions using DMEM with 10% FBS before recording. Serum-starved cells were cultured for 24 hr before recording in DMEM with no added FBS. In this and subsequent figures, filled symbols were used for currents from serum-fed cells, and open symbols were used for currents from serum-starved cells. Currents were normalized to peak for both panels. A, Rapid application currents elicited from serum-fed and serum-starved cells are overlaid for comparison. Desensitization was decreased, and deactivation was accelerated in serum-starved cells. B, PKI (50 μg/ml) was included in the intracellular recording pipette of some lifted cells, which were grown under standard serum-fed culture conditions. PKI reduced desensitization and accelerated deactivation in a manner similar to serum-starved cells.
Figure 3.
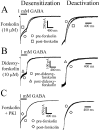
Modulation of α1β3γ2L currents by forskolin. GABA was applied to lifted HEK293T cells expressing α1β3γ2L GABA receptors. A, GABA (1 m
m
) was applied for 400 msec for all traces. After formation of a gigaohm seal, each cell was lifted from the bottom of the dish, and GABA was applied as an initial control (pre-forskolin). After the control GABA application, the lifted cell was incubated in forskolin (10 μ
m
) for 5 min and was then washed for 1 sec in external bath solution before another 400 msec GABA application (post-forskolin). The extent of current desensitization was greater after incubation in forskolin (left). The right panel shows the same deactivation shown on the left, except the amount of current at the time of GABA offset is normalized to aid comparison. Deactivation was accelerated after forskolin application. B, No change in desensitization or deactivation was seen after dideoxyforskolin application. C, When the forskolin paradigm in A was repeated with PKI (50 μg/ml) in the intracellular recording pipette, no changes in desensitization or deactivation were observed.
Figure 4.
Mutation of the β1 subtype serine 409 to alanine decreased desensitization and accelerated deactivation. Wild-type α1β1γ2L receptor currents were compared with α1β1(S409A)γ2L receptor currents. A, Currents evoked by a 400 msec application of GABA (1 m
m
) to α1β1γ2L and α1β1(S409A)γ2L receptors were normalized to peak current. α1β1(S409A)γ2L receptor currents had decreased desensitization and accelerated deactivation compared with currents from α1β1γ2L receptors. B, Currents evoked by a 6 sec application of GABA (1 m
m
) to α1β1γ2L and α1β1(S409A)γ2L receptors were normalized to peak current. α1β1(S409A)γ2L receptor currents had decreased desensitization and accelerated deactivation compared with currents from α1β1γ2L receptors. Desensitization of α1β1(S409A)γ2L receptor currents was greater than desensitization observed with α1β1γ2L receptor currents evoked by 400 msec and 6 sec GABA application. Deactivation after time of GABA offset was accelerated with α1β1(S409A)γ2L receptor currents compared with α1β1γ2L receptor currents for evoked by 400 msec and 6 sec GABA application.
Figure 5.
Mutation of β3 (S407A,S408A) decreased desensitization and accelerated deactivation, whereas β3(S407E, S408E)-containing receptors did not differ from wild type. Wild-type α1β3γ2L receptors were compared with α1β3(S407A,S408A)γ2L (β3AA receptors), α1β3(S407E,S408A)γ2L (β3EA), α1β3(S407A,S408E)γ2L (β3AE), and α1β3(S407E,S408E)γ2L (β3EE receptors) mutant receptors. Currents were normalized to peak. Initially, we compared β3AA receptors and β3EE receptors with wild type. A 400 msec GABA (1 m
m
) application to β3AA and β3EA receptors resulted in a decreased desensitization and accelerated deactivation compared with wild-type, β3EE, and β3AE GABA receptors. A 6 sec GABA (1 m
m
) application to β3AA and β3EA receptors resulted in an decreased desensitization and accelerated deactivation compared with wild-type β3EE, and β3AE GABA receptors.
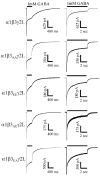
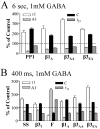
Figure 6.
Altering the β phosphorylation site primarily altered τ1, A1, C, and τD. GABA (1 m
m
) was applied to lifted cells for 6 sec (A) or 400 msec (B). Selected kinetic parameters (τ1, A1, C, and τD) were expressed as a mean percentage of the value of the appropriate β subunit control. Control was defined as 100% and is indicated by a dotted line. In the case of forskolin, the percentage was calculated using the mean of the paired controls recorded before forskolin application. Only groups with significant differences from control were displayed. Groups transfected with α1β3γ2L GABAA receptors include PP1, serum starved (SS), PKI treated (β3I), forskolin treated (F), β3(S407A, S408A), and β3(S407E, S408A). One group transfected with α1β1γ2L GABAA receptors is the β1(S409A) mutant (β1A). All data displayed were significantly different from control, except for the A1 parameter for PP1 (A) and τ1 and A1 for β3I (supplemental Tables A and B).
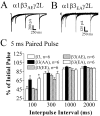
Figure 7.
Brief (5 msec) GABA application response durations were modulated by mutation of β3 subtype serine 407 and 408 to alanine. GABA (1 m
m
) was applied to excised macropatches for 5 msec. A, Representative data traces are displayed for α1β3γ2L, α1β3(S407A,S408A)γ2L (β3AA), and α1β3(S407A,S408A)γ2L (β3EE) receptors. B, Data from A are normalized to peak and overlaid for comparison showing faster deactivation for β3AA receptors and slower deactivation for β3EE receptors compared with wild-type receptors. Deactivation was accelerated in β3AA receptor currents compared with β3EE and wild-type receptor currents. C, Representative data showing current deactivation after 400 msec and 6 sec GABA application. For purposes of comparison, the level of current at offset of GABA (arrow) is normalized, and most current in the presence of GABA has been excluded from the display. Deactivation was accelerated in β3AA receptor currents compared with β3EE receptor currents.
Figure 8.
Brief (5 msec) GABA repeated pulse inhibition was decreased by of β3 subtype serine 408 to alanine.GABA (1 m
m
) was applied for 5 msec twice in succession to excised macropatches at varying interpulse intervals (100 msec, 300 msec, 1 sec, 2 sec). Shown are representative data traces for α1β3(S407A,S408E)γ2L (β3AE) receptors (A) and α1β3(S407E,S408A)γ2L (β3EA) receptors (B). Each panel of representative data are four overlaid raw data traces aligned such that the initial currents were superimposed and each subsequent GABA current represented the second pulse of repeated pulse protocols. Each second pulse represented a separate trial on the same macropatch. C, Repeated pulse inhibition was measured for α1β3γ2L (β3), α1β3(S407A,S408A)γ2L (β3AA), α1β3(S407E,S408E)γ2L (β3EE), α1β3(S407A,S408E)γ2L (β3AE), and α1β3(S407E,S408A)γ2L (β3EA) receptors and was displayed as the percentage of second pulse peak to initial pulse peak (amplitudesecond current/amplitudeinitial current × 100). Repeated pulse inhibition was decreased for β3AA (66.9%) and β3EA (69.3%) receptors at a 100 msec interpulse interval compared with wild-type (46.2%), β3EE (47.6%), and β3AE (44.0%) receptors.
Similar articles
- Adjacent phosphorylation sites on GABAA receptor beta subunits determine regulation by cAMP-dependent protein kinase.
McDonald BJ, Amato A, Connolly CN, Benke D, Moss SJ, Smart TG. McDonald BJ, et al. Nat Neurosci. 1998 May;1(1):23-8. doi: 10.1038/223. Nat Neurosci. 1998. PMID: 10195104 - D(1) dopamine receptor activation reduces GABA(A) receptor currents in neostriatal neurons through a PKA/DARPP-32/PP1 signaling cascade.
Flores-Hernandez J, Hernandez S, Snyder GL, Yan Z, Fienberg AA, Moss SJ, Greengard P, Surmeier DJ. Flores-Hernandez J, et al. J Neurophysiol. 2000 May;83(5):2996-3004. doi: 10.1152/jn.2000.83.5.2996. J Neurophysiol. 2000. PMID: 10805695 - Slow phases of GABA(A) receptor desensitization: structural determinants and possible relevance for synaptic function.
Bianchi MT, Macdonald RL. Bianchi MT, et al. J Physiol. 2002 Oct 1;544(Pt 1):3-18. doi: 10.1113/jphysiol.2002.020255. J Physiol. 2002. PMID: 12356876 Free PMC article. - GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts.
Haas KF, Macdonald RL. Haas KF, et al. J Physiol. 1999 Jan 1;514 ( Pt 1)(Pt 1):27-45. doi: 10.1111/j.1469-7793.1999.027af.x. J Physiol. 1999. PMID: 9831714 Free PMC article. - Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAa receptors.
Bianchi MT, Haas KF, Macdonald RL. Bianchi MT, et al. J Neurosci. 2001 Feb 15;21(4):1127-36. doi: 10.1523/JNEUROSCI.21-04-01127.2001. J Neurosci. 2001. PMID: 11160383 Free PMC article.
Cited by
- Cross talk between synaptic receptors mediates NMDA-induced suppression of inhibition.
Chisari M, Zorumski CF, Mennerick S. Chisari M, et al. J Neurophysiol. 2012 May;107(9):2532-40. doi: 10.1152/jn.01145.2011. Epub 2012 Jan 25. J Neurophysiol. 2012. PMID: 22279196 Free PMC article. - Modes and models of GABA(A) receptor gating.
Lema GM, Auerbach A. Lema GM, et al. J Physiol. 2006 Apr 1;572(Pt 1):183-200. doi: 10.1113/jphysiol.2005.099093. Epub 2006 Feb 2. J Physiol. 2006. PMID: 16455693 Free PMC article. - Tandem subunits effectively constrain GABAA receptor stoichiometry and recapitulate receptor kinetics but are insensitive to GABAA receptor-associated protein.
Boileau AJ, Pearce RA, Czajkowski C. Boileau AJ, et al. J Neurosci. 2005 Dec 7;25(49):11219-30. doi: 10.1523/JNEUROSCI.3751-05.2005. J Neurosci. 2005. PMID: 16339017 Free PMC article. - Phenols and GABAA receptors: from structure and molecular mechanisms action to neuropsychiatric sequelae.
Menzikov SA, Zaichenko DM, Moskovtsev AA, Morozov SG, Kubatiev AA. Menzikov SA, et al. Front Pharmacol. 2024 Jan 18;15:1272534. doi: 10.3389/fphar.2024.1272534. eCollection 2024. Front Pharmacol. 2024. PMID: 38303988 Free PMC article. Review. - Pathophysiology of and therapeutic options for a GABRA1 variant linked to epileptic encephalopathy.
Bai YF, Chiu M, Chan ES, Axerio-Cilies P, Lu J, Huh L, Connolly MB, Guella I, Farrer MJ, Xu ZD, Liu L, Demos M, Wang YT. Bai YF, et al. Mol Brain. 2019 Nov 10;12(1):92. doi: 10.1186/s13041-019-0513-9. Mol Brain. 2019. PMID: 31707987 Free PMC article.
References
- Angelotti TP, Uhler MD, Macdonald RL ( 1993) Enhancement of recombinant gamma-aminobutyric acid type A receptor currents by chronic activation of cAMP-dependent protein kinase. Mol Pharmacol 44: 1202-1210. - PubMed
- Axon Instruments ( 1999) Principles of operation. In: Axopatch 200B patch clamp theory and operation, pp 75-101. Union City, CA: Axon Instruments.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous