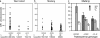A human complement receptor 1 polymorphism that reduces Plasmodium falciparum rosetting confers protection against severe malaria - PubMed (original) (raw)
A human complement receptor 1 polymorphism that reduces Plasmodium falciparum rosetting confers protection against severe malaria
Ian A Cockburn et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Parasitized red blood cells (RBCs) from children suffering from severe malaria often adhere to complement receptor 1 (CR1) on uninfected RBCs to form clumps of cells known as "rosettes." Despite a well documented association between rosetting and severe malaria, it is controversial whether rosetting is a cause or a correlate of parasite virulence. CR1-deficient RBC show greatly reduced rosetting; therefore, we hypothesized that, if rosetting is a direct cause of malaria pathology, CR1-deficient individuals should be protected against severe disease. In this study, we show that RBC CR1 deficiency occurs in up to 80% of healthy individuals from the malaria-endemic regions of Papua New Guinea. This RBC CR1 deficiency is associated with polymorphisms in the CR1 gene and, unexpectedly, with alpha-thalassemia, a common genetic disorder in Melanesian populations. Analysis of a case-control study demonstrated that the CR1 polymorphisms and alpha-thalassemia independently confer protection against severe malaria. We have therefore identified CR1 as a new malaria resistance gene and provided compelling evidence that rosetting is an important parasite virulence phenotype that should be a target for drug and vaccine development.
Figures
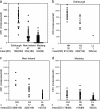
Fig. 1.
RBC CR1 deficiency is common in Melanesians and is associated with SNPs in the CR1 gene. (a) RBC CR1 levels in Edinburgh (United Kingdom), Madang (PNG), and New Ireland (PNG). Each point represents the mean RBC CR1 level of a single individual in molecules per cell. SD, standard deviation. (b_–_d) RBC CR1 levels in relation to CR1 genotype based on typing at the n3650 polymorphism in exon 22. H, high-expression allele; L, low-expression allele; na, not applicable.
Fig. 2.
RBC CR1 deficiency in Melanesians is associated with α-thalassemia. (a and b) RBC CR1 level in relation to α-thalassemia genotype. Thalassemia genotypes are the following: -α/αα, heterozygotes for the -α4.2 deletion (32); -α/-α, homozygotes for the -α4.2 deletion; and αα/αα, normal α-globin gene structure. Only one individual carried the -α3.7 deletion (32); therefore, this case was excluded from the analysis. (c) The effect of α-thalassemia and CR1 exon 22 genotype on RBC CR1 level are independent of each other. The mean CR1 level for the Madang population subdivided by CR1 genotype (n3650 polymorphism) and α-thalassemia (-α4.2 deletion) is shown, and standard errors are indicated. The HH/normal α-globin genotype was not represented.
Similar articles
- Mapping of the region of complement receptor (CR) 1 required for Plasmodium falciparum rosetting and demonstration of the importance of CR1 in rosetting in field isolates.
Rowe JA, Rogerson SJ, Raza A, Moulds JM, Kazatchkine MD, Marsh K, Newbold CI, Atkinson JP, Miller LH. Rowe JA, et al. J Immunol. 2000 Dec 1;165(11):6341-6. doi: 10.4049/jimmunol.165.11.6341. J Immunol. 2000. PMID: 11086071 - Red blood cell complement receptor one level varies with Knops blood group, α(+)thalassaemia and age among Kenyan children.
Opi DH, Uyoga S, Orori EN, Williams TN, Rowe JA. Opi DH, et al. Genes Immun. 2016 Apr;17(3):171-8. doi: 10.1038/gene.2016.2. Epub 2016 Feb 4. Genes Immun. 2016. PMID: 26844958 Free PMC article. - Human complement receptor type 1 (CR1) binds to a major malarial adhesin.
Krych-Goldberg M, Moulds JM, Atkinson JP. Krych-Goldberg M, et al. Trends Mol Med. 2002 Nov;8(11):531-7. doi: 10.1016/s1471-4914(02)02419-x. Trends Mol Med. 2002. PMID: 12421687 Review. - Three Is a Crowd - New Insights into Rosetting in Plasmodium falciparum.
Yam XY, Niang M, Madnani KG, Preiser PR. Yam XY, et al. Trends Parasitol. 2017 Apr;33(4):309-320. doi: 10.1016/j.pt.2016.12.012. Epub 2017 Jan 18. Trends Parasitol. 2017. PMID: 28109696 Review.
Cited by
- Comparison of rapid diagnostic test Plasmotec Malaria-3, microscopy, and quantitative real-time PCR for diagnoses of Plasmodium falciparum and Plasmodium vivax infections in Mimika Regency, Papua, Indonesia.
Fransisca L, Kusnanto JH, Satoto TB, Sebayang B, Supriyanto, Andriyan E, Bangs MJ. Fransisca L, et al. Malar J. 2015 Mar 5;14:103. doi: 10.1186/s12936-015-0615-5. Malar J. 2015. PMID: 25890368 Free PMC article. - Dantu Blood Group Erythrocytes Form Large Plasmodium falciparum Rosettes Less Commonly.
Carlier MSA, Nyamu W, Makale J, Williams TN, Rowe JA, Kariuki SN. Carlier MSA, et al. Am J Trop Med Hyg. 2024 Jan 30;110(3):436-443. doi: 10.4269/ajtmh.23-0347. Print 2024 Mar 6. Am J Trop Med Hyg. 2024. PMID: 38295409 Free PMC article. - How malaria has affected the human genome and what human genetics can teach us about malaria.
Kwiatkowski DP. Kwiatkowski DP. Am J Hum Genet. 2005 Aug;77(2):171-92. doi: 10.1086/432519. Epub 2005 Jul 6. Am J Hum Genet. 2005. PMID: 16001361 Free PMC article. Review. - A paradoxical population structure of var DBLα types in Africa.
Tan MH, Tiedje KE, Feng Q, Zhan Q, Pascual M, Shim H, Chan YB, Day KP. Tan MH, et al. PLoS Pathog. 2025 Feb 4;21(2):e1012813. doi: 10.1371/journal.ppat.1012813. eCollection 2025 Feb. PLoS Pathog. 2025. PMID: 39903780 Free PMC article. - Complement in cancer: untangling an intricate relationship.
Reis ES, Mastellos DC, Ricklin D, Mantovani A, Lambris JD. Reis ES, et al. Nat Rev Immunol. 2018 Jan;18(1):5-18. doi: 10.1038/nri.2017.97. Epub 2017 Sep 18. Nat Rev Immunol. 2018. PMID: 28920587 Free PMC article. Review.
References
- Breman, J. G. (2001) Am. J. Trop. Med. Hyg. 64, 1-11. - PubMed
- David, P. H., Handunnetti, S. M., Leech, J. H., Gamage, P. & Mendis, K. N. (1988) Am. J. Trop. Med. Hyg. 38, 289-297. - PubMed
- Kaul, D. K., Roth, E. F. J., Nagel, R. L., Howard, R. J. & Handunnetti, S. M. (1991) Blood 78, 812-819. - PubMed
- Nash, G. B., Cooke, B. M., Carlson, J. & Wahlgren, M. (1992) Br. J. Haematol. 82, 757-763. - PubMed
- Carlson, J., Helmby, H., Hill, A. V., Brewster, D., Greenwood, B. M. & Wahlgren, M. (1990) Lancet 336, 1457-1460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases