Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds - PubMed (original) (raw)
Comparative Study
Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds
Lari Häkkinen et al. Am J Pathol. 2004 Jan.
Abstract
Integrin alphavbeta6 is an epithelial cell-specific receptor that is not normally expressed by resting epithelium but its expression is induced during wound healing. The function of alphavbeta6-integrin in wound repair is not clear. In the present study, we showed that beta6-integrin expression was strongly up-regulated in the epidermis in human chronic wounds but not in different forms of skin fibrosis. To test whether increased beta6-integrin expression plays a role in abnormal wound healing we developed four homozygous transgenic mouse lines that constitutively expressed human beta6-integrin in the epithelium. The mice developed normally and did not show any histological abnormalities in the skin. The rate of experimental skin wound closure was unaltered and the wounds healed without significant scar formation. However, during breeding program 16.1 to 27.0% of transgenic mice developed spontaneous, progressing fibrotic chronic ulcers. None of the wild-type animals developed these lesions. The chronic lesions had areas with severe fibrosis and numerous activated macrophages and fibroblasts expressing transforming growth factor (TGF)-beta. The level of TGF-beta1 was significantly increased in the lesions as compared with normal skin. The findings suggest that increased alphavbeta6-integrin in keratinocytes plays an active part in abnormal wound healing possibly through a mechanism involving increased activation of TGF-beta.
Figures
Figure 1
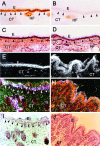
Expression of β6-integrin mRNA (A, D, G) and immunolocalization of type I procollagen (B, E, H) in chronic human skin wounds. Arrowheads indicate epidermal-dermal junction and arrows indicate fibroblasts that show positive immunoreactivity for type I procollagen. Integrin β6 mRNA is most abundantly localized at the basal epithelial cells. At close proximity to these cells fibroblasts show strong immunoreactivity to type I procollagen. Occasionally, nonspecific intracellular staining in keratinocytes was noted in immunostainings with type I collagen antibody (B, E, and H). A–C: Diabetic ulcer; D–E: decubitus ulcer; G–I: chronic leg ulcer. E, Epithelium; CT, connective tissue; FC, fibrin clot. Asterisk, Collagen fibers. A, D, G: Dark-field image; C, F, I: H&E staining.
Figure 2
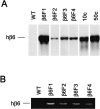
Southern blotting of mouse genomic DNA for human β_6-integrin_ gene (A) and reverse transcriptase-polymerase chain reaction detection of human β6 mRNA from newborn mouse skin (B). WT, Wild-type mice; β6F1 to β6F4, four homozygous hβ6-integrin-expressing mouse lines. In A, 10c and 50c indicate β_6-integrin_ cDNA standards for 10 and 50 gene copies, respectively.
Figure 3
Localization of human β6-integrin mRNA (G and H) and immunolocalization of β6-integrin (A and B), cytokeratin 14 (C and D), and αv integrin subunit (E and F) in human β6-integrin-transgenic (hβ6F1) (A, C, E, G, I) and wild-type (B, D, F, H, J) mouse skin. I and J: H&E staining of the corresponding samples as in G and H. Arrowheads indicate epidermal-dermal junction. Strong expression of hβ6-integrin mRNA and immunoreactivity for β6-integrin localizes at the basal epithelial cells and hair follicles of transgenic mouse skin. Integrin β6 expression and protein co-localizes with endogenous αv integrin subunit and cytokeratin 14 at the basal epithelial cells. Wild-type animals do not express β6-integrin except for weak immunoreactivity at the outer root sheath cells of hair follicles. E, Epithelium; CT, connective tissue; HF, hair follicle.
Figure 4
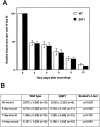
Cutaneous wound healing in wild-type and human β6-integrin-transgenic mouse skin. A: Quantitation of wound surface areas at different time points after wounding in wild-type (WT) and in a transgenic (β6F1) mouse line. B: Breaking strength (load in Newtons at breaking point) of incisional wounds and nonwounded skin in wild-type and human β6-integrin-transgenic mice (hβ6F1) at different time points after wounding. There were no differences in wound closure rate (wound surface area at different time points) or in the wound breaking strength between wild-type and human β6-integrin-transgenic mice.
Figure 5
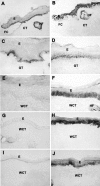
Histological characterization of cutaneous wound healing in wild-type (A, C, E, G) and human β6-integrin-transgenic mouse (β6F1) skin (B, D, F, H) at 3 (A and B), 7 (C and D), 30 (E and F), and 44 (G and H) days after wounding. I and J: Histological sections of nonwounded skin in wild-type and in the β6-integrin-transgenic mouse, respectively. Histologically, both wild-type and transgenic animals showed similar wound healing response with complete re-epithelialization at day 7 and formation of collagen-rich wound connective tissue by day 30 after wounding. A–D and I and J: H&E staining; E–H: phosphotungstic acid hematoxylin staining. E, Epithelium; CT, connective tissue; GT, granulation tissue; WCT, wound connective tissue.
Figure 6
Immunohistochemical localization of β6-integrin in cutaneous wound healing in wild-type (A, C, E, G, I) and human β6-integrin-transgenic mouse (hβ6F1) skin (B, D, F, H, J). Results show β6-integrin expression at 3 (A and B), 7 (C and D), 14 (E and F), 28 (G and H), and 44 (I and J) days after wounding. E, Epithelium; FC, fibrin clot; CT, connective tissue; GT, granulation tissue; WCT, wound connective tissue.
Figure 7
A: Representative clinical appearance of a chronic progressing wound in a human β6-integrin-transgenic (hβ6F1) mouse. a: Early lesion; b: progressed lesion; c: advanced lesion showing progression to the abdominal area. B: Prevalence of skin lesions in the wild-type and human β6-integrin-transgenic mice. 1Males; 2females; 3affected: Mice with progressing scarring and/or chronic wounds in the lower back; 4percent of affected mice of total number of mice or percent of affected males or females of total number of males or females, respectively; 5mean age of the mice in weeks. WT, wild-type mice; hβ6F1, hβ6F2, hβ6F3, hβ6F4 transgenic mouse lines.
Figure 8
Histological characterization of superficial ulcer areas in representative chronic, progressing lesions from human β6-integrin-transgenic mice. A, C, E, and G: H&E staining; B, D, F, H: Phosphotungstic acid hematoxylin staining. A and B: Normal healthy skin from human β6-integrin-transgenic mouse (hβ6F1). C–F: Edge of an open ulceration in a chronic, progressing wound from hβ6F1 (C and D) and hβ6F2 (E and F) human β6-integrin-transgenic mice. The connective tissue next to the ulcer is strongly thickened and shows abundant collagen accumulation (asterisk in D and F). The epithelium next to the ulceration can be thickened (C and D) and/or show hyperkeratosis (E and F). G and H: Mid-ulcer area from the chronic, progressing wound from the hβ6F2 mouse. The connective tissue beneath the superficial ulceration is heavily infiltrated with inflammatory cells. Arrowhead, Open ulcer edge; E, epithelium; CT, connective tissue; HF, hair follicle; SC, scar tissue; ICT, inflamed connective tissue.
Figure 9
Immunohistochemical characterization of representative areas next to the superficial ulcers in chronic, progressing wound samples from a human β6-integrin-transgenic mouse (hβ6F1). A: H&E staining; B: Phosphotungstic acid hematoxylin staining; C: in situ hybridization of human β6-integrin (dark-field image); D: H&E illustration of the same sample as in C; E: immunolocalization of β6-integrin; F: immunolocalization of TGF-β1, -2, and -3; G: immunolocalization of α-smooth muscle actin; H: immunostaining of macrophages. E, Epithelium; SC, scar tissue; HF, hair follicle.
Figure 10
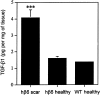
Level (pg/mg of tissue) of total TGF-β1 (active + latent) in chronic scars from β6-integrin-overexpressing mice (hβ6 scar; β6F1, n = 3 and β6F2, n = 3) and in healthy skin from β6-integrin-overexpressing (hβ6 healthy; β6F1, n = 3 and β6F2, n = 3) and from wild-type mice (WT healthy, n = 8) was analyzed with the TGF-β1 immunoassay. The amount of TGF-β1 in chronic scars from transgenic animals was significantly higher as compared with normal skin from transgenic and control animals (P < 0.001, analysis of variance).
Similar articles
- Mice lacking beta6 integrin in skin show accelerated wound repair in dexamethasone impaired wound healing model.
Xie Y, Gao K, Häkkinen L, Larjava HS. Xie Y, et al. Wound Repair Regen. 2009 May-Jun;17(3):326-39. doi: 10.1111/j.1524-475X.2009.00480.x. Wound Repair Regen. 2009. PMID: 19660040 - Expression of integrin alphavbeta6 and TGF-beta in scarless vs scar-forming wound healing.
Eslami A, Gallant-Behm CL, Hart DA, Wiebe C, Honardoust D, Gardner H, Häkkinen L, Larjava HS. Eslami A, et al. J Histochem Cytochem. 2009 Jun;57(6):543-57. doi: 10.1369/jhc.2009.952572. Epub 2009 Feb 16. J Histochem Cytochem. 2009. PMID: 19223298 Free PMC article. - The alphavbeta6 integrin plays a role in compromised epidermal wound healing.
AlDahlawi S, Eslami A, Häkkinen L, Larjava HS. AlDahlawi S, et al. Wound Repair Regen. 2006 May-Jun;14(3):289-97. doi: 10.1111/j.1743-6109.2006.00123.x. Wound Repair Regen. 2006. PMID: 16808807 - Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis.
Lichtman MK, Otero-Vinas M, Falanga V. Lichtman MK, et al. Wound Repair Regen. 2016 Mar;24(2):215-22. doi: 10.1111/wrr.12398. Epub 2016 Mar 2. Wound Repair Regen. 2016. PMID: 26704519 Review.
Cited by
- Connective tissue growth factor causes EMT-like cell fate changes in vivo and in vitro.
Sonnylal S, Xu S, Jones H, Tam A, Sreeram VR, Ponticos M, Norman J, Agrawal P, Abraham D, de Crombrugghe B. Sonnylal S, et al. J Cell Sci. 2013 May 15;126(Pt 10):2164-75. doi: 10.1242/jcs.111302. Epub 2013 Mar 22. J Cell Sci. 2013. PMID: 23525012 Free PMC article. - Interplay between Cell-Surface Receptors and Extracellular Matrix in Skin.
Kleiser S, Nyström A. Kleiser S, et al. Biomolecules. 2020 Aug 11;10(8):1170. doi: 10.3390/biom10081170. Biomolecules. 2020. PMID: 32796709 Free PMC article. Review. - Transforming growth factor-β in myocardial disease.
Frangogiannis NG. Frangogiannis NG. Nat Rev Cardiol. 2022 Jul;19(7):435-455. doi: 10.1038/s41569-021-00646-w. Epub 2022 Jan 4. Nat Rev Cardiol. 2022. PMID: 34983937 Review. - Role of platelet factor 4 in arteriovenous fistula maturation failure: What do we know so far?
Xiao Y, Vazquez-Padron RI, Martinez L, Singer HA, Woltmann D, Salman LH. Xiao Y, et al. J Vasc Access. 2024 Mar;25(2):390-406. doi: 10.1177/11297298221085458. Epub 2022 Jun 24. J Vasc Access. 2024. PMID: 35751379 Free PMC article. Review. - The Potential Role of a Surface-Modified Additive-Manufactured Healing Abutment on the Expression of Integrins α2, β1, αv, and β6 in the Peri-Implant Mucosa: A Preliminary Human Study.
Roth LA, Bastos MF, Melo MA, Barão VAR, Costa RC, Giro G, Souza JGS, Grzech-Leśniak K, Shibli JA. Roth LA, et al. Life (Basel). 2022 Jun 22;12(7):937. doi: 10.3390/life12070937. Life (Basel). 2022. PMID: 35888027 Free PMC article.
References
- Garner WL. Epidermal regulation of dermal fibroblast activity. Plast Reconstr Surg. 1998;102:135–139. - PubMed
- Bauer BS, Tredget EE, Marcoux Y, Scott PG, Ghahary A. Latent and active transforming growth factor beta1 released from genetically modified keratinocytes modulates extracellular matrix expression by dermal fibroblasts in a coculture system. J Invest Dermatol. 2002;119:456–463. - PubMed
- Nowinski D, Hoijer P, Engstrand T, Rubin K, Gerdin B, Ivarsson M. Keratinocytes inhibit expression of connective tissue growth factor in fibroblasts in vitro by an interleukin-1alpha-dependent mechanism. J Invest Dermatol. 2002;119:449–455. - PubMed
- Carroll JM, Romero MR, Watt FM. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 1995;83:957–968. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases