The alpha- and beta'-COP WD40 domains mediate cargo-selective interactions with distinct di-lysine motifs - PubMed (original) (raw)
The alpha- and beta'-COP WD40 domains mediate cargo-selective interactions with distinct di-lysine motifs
Anne Eugster et al. Mol Biol Cell. 2004 Mar.
Abstract
Coatomer is required for the retrieval of proteins from an early Golgi compartment back to the endoplasmic reticulum. The WD40 domain of alpha-COP is required for the recruitment of KKTN-tagged proteins into coatomer-coated vesicles. However, lack of the domain has only minor effects on growth in yeast. Here, we show that the WD40 domain of beta'-COP is required for the recycling of the KTKLL-tagged Golgi protein Emp47p. The protein is degraded more rapidly in cells with a point mutation in the WD40 domain of beta'-COP (sec27-95) or in cells lacking the domain altogether, whereas a point mutation in the Clathrin Heavy Chain Repeat (sec27-1) does not affect the turnover of Emp47p. Lack of the WD40 domain of beta'-COP has only minor effects on growth of yeast cells; however, absence of both WD40 domains of alpha- and beta'-COP is lethal. Two hybrid studies together with our analysis of the maturation of KKTN-tagged invertase and the turnover of Emp47p in alpha- and beta'-COP mutants suggest that the two WD40 domains of alpha- and beta'-COP bind distinct but overlapping sets of di-lysine signals and hence both contribute to recycling of proteins with di-lysine signals.
Figures
Figure 1.
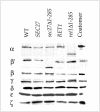
(A) Schematic of the domain structure of α- and β′-COP. In this study, we show that the WD40 domain of β′-COP (residues 1–285) is nonessential but required for selective interactions with a KTKLL motif, whereas the α-COP WD40 domain interacts with the “classical” KKTN-motif (Eugster et al., 2000). A carboxy-terminal region of β′-COP, defined by sec27-1 (a G688D mutation) is involved in maintaining α-COP stability and coatomer integrity (see text for details). (B) Growth phenotype of α- and β′-COP mutants harboring a deletion of the respective WD40 domain. Cells were streaked onto YEPD plates and grown for 3 d at the temperatures indicated. Strains were as indicated: wild-type control strains CEN::RET1, CEN::SEC27 and mutant strains _CEN::ret1_Δ1-285 and _CEN::sec27_Δ1-285. Note that _sec27_Δ1-285 cells are strongly temperature sensitive.
Figure 2.
Levels of coatomer subunits in _sec27_Δ1-285 cells are normal. Whole cell extracts of _ret1_Δ1-285, _sec27_Δ1-285, control cells (CEN::RET1 and CEN::SEC27), and wild-type cells (YPH500) grown at 24°C were analyzed by immunoblot on equal OD600 equivalents of cells. Note the difference in molecular weight between wild-type and truncated α-COP or β′-COP. Further note that all other subunits are present in comparable amounts in all strains.
Figure 3.
A strain lacking the WD40 domains of both α-COP and β′-COP is inviable. The mutant strains _ret1_Δ1-285 + pCEN::RET1 (URA3), _sec27_Δ1-285 +pCEN::SEC27 (URA3), _ret1_Δ::HIS3/sec27Δ::LEU2 +pCEN::ret1Δ1–285 +pCEN::sec27Δ1-285 +pCEN::SEC27 (URA3) and congenic control strains CEN::RET1 +pCEN::RET1 (URA3), CEN::SEC27 +pCEN::SEC27 (URA3), and _ret1_Δ::HIS3/sec27Δ::LEU2 +pCEN::ret1Δ1–285 +pCEN:: SEC27 +pCEN::SEC27 (URA3) were streaked out onto 5-FOA plates to induce loss of the URA3 plasmids, and incubated at 24°C for 4 d. Note that a _ret1_Δ1-285/_sec27_Δ1-285 strain is not viable without the pCEN::SEC27 plasmid.
Figure 4.
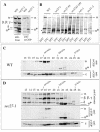
α-COP is destabilized in sec27-1 cells. (A and B) Identification of a ∼65-kDa α-COP fragment (α*) in sec27-1 cells and other coatomer mutants as indicated, and in wild-type cells (WT). For details, see text. (C) Superose 6 gel filtration of cytosolic proteins from temperature-shifted wild-type cells (WT). α-COP species were identified with the α-COP peptide antiserum, and ε-COP with a specific rabbit antiserum. (D) Superose 6 gel filtration of cytosolic proteins from sec27-1 cells. SDS-PAGE separated proteins of column fractions were probed with anti-coatomer antiserum to identify α-, β′-, β-, and γ-COP and the α-COP fragment (α*) (top). The antiserum against the carboxy-terminal α-COP peptide (“anti-α-COP”) was used (middle); the bottom panel visualizes ε-COP. Note the reduced signal for intact α-COP in fractions 18 + 19 from sec27-1 cells compared with wild type. Further note that a large fraction of α-COP immunoreactivity is present in fractions 22 + 23, corresponding to a partial complex of ∼200 kDa in which ε-COP cofractionates. An additional peak of monomeric ε-COP is seen in fraction 26. Fraction numbers and positions of marker proteins are indicated: thyroglobulin (669,000), β-amylase (200,000), and carboanhydrase (29,000).
Figure 5.
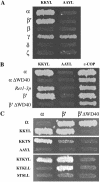
α- and β′-COP directly interact with variants of di-lysine motifs. (A) Coatomer subunits, denoted with Greek symbols, in the bait vector were tested against the KKYL or AAYL motifs in the prey vector. Note that both α- and β′-COP interact with the KKYL motif, in a manner that depends on the presence of the lysines. γ-COP displayed background and thus could not be analyzed. (B) The interaction of α- and β′-COP with the KKYL motif depends on the presence of their WD40 domains. As expected, the interaction of α- and β′-COP with ε-COP is unaffected by truncation of the WD40 domains. “αΔWD40” and “β'ΔWD40” correspond to _ret1_Δ1-285 and _sec27_Δ1-285 in the text. (C) α-COP, or the different di-lysine motifs, in the bait vector were tested against α-COP, β′-COP, or the β′ΔWD40 mutant in the prey vector. Note that the KKTN motif interacts with α-COP and the KTKLL motif with β′-COP. The presence of the WD40 domain on β′-COP is required for the interaction with KTKLL, but not with α-COP. Results shown are from the growth assay on minimal plates lacking leucine.
Figure 6.
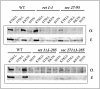
Emp47p is destabilized in β′-COP WD40 domain mutants. (A) Degradation of Emp47p measured by immunoblotting in the strains as indicated; for details, see text. Note a significant reduction of Emp47p levels in cells of the β′-COP WD40 domain mutants sec27-95 and _sec27_Δ1-285, but not in sec27-1 cells or ret1-1 and _ret1_Δ1-285 cells. (B) wild-type (wt), sec27-1 and sec27-95 cells were pulsed for 20 min at 24°C and chased for the times indicated, and Emp47p levels were quantitated. Note a dramatic reduction of Emp47p levels in sec27-95 cells but not sec27-1 cells at 120 min. (C) Turnover of Emp47p in α- and β′-COP WD40 domain mutants. The experiment was performed as in B, by using wild-type cells and the _sec27_Δ1-285, _sec27-95, ret1_Δ1-285, ret1-1 mutants. Curves represent PhosphorImager quantitation of a representative experiment. Note the reduction of Emp47p levels in _sec27_Δ1-285 and sec27-95 cells but not in the α-COP mutants.
Figure 7.
(A) The β′-COP WD40 domain mutants _sec27_Δ1-285 and sec27-95 behave like wild-type for KKTN trafficking in vivo. _sec27_Δ1-285 and sec27-95 cells and _ret1_Δ1-285 and wild-type (CEN::SEC27) cells as controls, all expressing the Wbp1-Inv.-KKTN fusion construct, were grown at 24°C. Before the experiment cells were preshifted to 30°C for 3 h and then pulse labeled at 30°C, and chased for 0 and 1 h at 30°C. Intact and PEP4 processed bands migrate at 70 and 56 kDa, respectively. The percentage of processing after 1 h was quantified using a PhosphorImager. (B) Same as in A, except that _ret1_Δ1-285, _sec27_Δ1-285, and control (CEN::SEC27) cells were grown at 24°C, pulse labeled for 20 min at 24°C, and chased for 0 and 1 h at 24°C. Note that _ret1_Δ1-285 cells show a strong KKTN retrieval defect at both 24 and 30°C, whereas _sec27_Δ1-285 cells behave like wild-type. (C) _sec27_Δ1-285 and sec27-95 cells display a kinetic delay in CPY transport at permissive temperature. Analysis of CPY transport in _sec27_Δ1-285, _sec27-95, ret1_Δ1-285, ret1-1, and wild-type (WT) cells. Cells were radiolabeled for 10 min at 24°C and chased for 0, 15, and 60 min. p1, p2, and mature forms of CPY are indicated, and processing to the mature form is quantified.
Figure 8.
Coatomer from WD40 domain truncation mutants is unable to bind to either the KKTN- or KTKLL motifs in vitro. Whole cell lysate of wild-type cells (WT), or mutants (_ret1_Δ1-285 cells, ret1-1 cells, _sec27-95, sec27-1, sec27_Δ1-285) was incubated for 2 h at 4°C with immobilized GST fusion proteins harboring the KKTN- or KTKLL motifs, or mutated motifs in which the lysines were changed to serine, SSTN or STSLL. Bound proteins eluted from the beads were analyzed by immunoblot. Note the expected binding pattern of coatomer from wild-type and ret1-1 mutant cells, and the absence of binding for coatomer from the α- and β′-COP WD40 domain truncation mutants. Coatomer from sec27-95 cells bound only weakly to KTKLL and not at all to the KKTN motif.
Similar articles
- Alpha-COP can discriminate between distinct, functional di-lysine signals in vitro and regulates access into retrograde transport.
Schröder-Köhne S, Letourneur F, Riezman H. Schröder-Köhne S, et al. J Cell Sci. 1998 Dec;111 ( Pt 23):3459-70. doi: 10.1242/jcs.111.23.3459. J Cell Sci. 1998. PMID: 9811561 - The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1-1 mutation in alpha-COP.
Schröder S, Schimmöller F, Singer-Krüger B, Riezman H. Schröder S, et al. J Cell Biol. 1995 Nov;131(4):895-912. doi: 10.1083/jcb.131.4.895. J Cell Biol. 1995. PMID: 7490292 Free PMC article. - COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP.
Eugster A, Frigerio G, Dale M, Duden R. Eugster A, et al. EMBO J. 2000 Aug 1;19(15):3905-17. doi: 10.1093/emboj/19.15.3905. EMBO J. 2000. PMID: 10921873 Free PMC article. - Making COPII coats.
Kirchhausen T. Kirchhausen T. Cell. 2007 Jun 29;129(7):1251-2. doi: 10.1016/j.cell.2007.06.015. Cell. 2007. PMID: 17604713 Review. - COP-coated vesicles in intracellular protein transport.
Harter C. Harter C. FEBS Lett. 1995 Aug 1;369(1):89-92. doi: 10.1016/0014-5793(95)00621-f. FEBS Lett. 1995. PMID: 7641892 Review.
Cited by
- Rules for the recognition of dilysine retrieval motifs by coatomer.
Ma W, Goldberg J. Ma W, et al. EMBO J. 2013 Apr 3;32(7):926-37. doi: 10.1038/emboj.2013.41. Epub 2013 Mar 12. EMBO J. 2013. PMID: 23481256 Free PMC article. - Mycovirus cryphonectria hypovirus 1 elements cofractionate with trans-Golgi network membranes of the fungal host Cryphonectria parasitica.
Jacob-Wilk D, Turina M, Van Alfen NK. Jacob-Wilk D, et al. J Virol. 2006 Jul;80(13):6588-96. doi: 10.1128/JVI.02519-05. J Virol. 2006. PMID: 16775345 Free PMC article. - Systematic analysis of complex genetic interactions.
Kuzmin E, VanderSluis B, Wang W, Tan G, Deshpande R, Chen Y, Usaj M, Balint A, Mattiazzi Usaj M, van Leeuwen J, Koch EN, Pons C, Dagilis AJ, Pryszlak M, Wang ZY, Hanchard J, Riggi M, Xu K, Heydari H, San Luis BJ, Shuteriqi E, Zhu H, Van Dyk N, Sharifpoor S, Costanzo M, Loewith R, Caudy A, Bolnick D, Brown GW, Andrews BJ, Boone C, Myers CL. Kuzmin E, et al. Science. 2018 Apr 20;360(6386):eaao1729. doi: 10.1126/science.aao1729. Science. 2018. PMID: 29674565 Free PMC article. - Diversity, origin, and evolution of the ESCRT systems.
Makarova KS, Tobiasson V, Wolf YI, Lu Z, Liu Y, Zhang S, Krupovic M, Li M, Koonin EV. Makarova KS, et al. mBio. 2024 Mar 13;15(3):e0033524. doi: 10.1128/mbio.00335-24. Epub 2024 Feb 21. mBio. 2024. PMID: 38380930 Free PMC article. - Effects of mutations in the WD40 domain of α-COP on its interaction with the COPI coatomer in Saccharomyces cerevisiae.
Kim KH, Kim EK, Jeong KY, Park YH, Park HM. Kim KH, et al. J Microbiol. 2012 Apr;50(2):256-62. doi: 10.1007/s12275-012-1326-z. Epub 2012 Apr 27. J Microbiol. 2012. PMID: 22538654
References
- Barlowe, C. (2003). Molecular recognition of cargo by the COP II complex: a most accommodating coat. Cell 114, 395-399. - PubMed
- Conibear, E., and Stevens, T.H. (1998). Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim. Biophys. Acta 1404, 211-230. - PubMed
- Cosson, P., and Letourneur, F. (1994). Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science 263, 1629-1631. - PubMed
- Donaldson, J.G., and Jackson, C.L. (2000). Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol. 12, 475-482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases