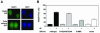Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair - PubMed (original) (raw)
Chk2 phosphorylation of BRCA1 regulates DNA double-strand break repair
Junran Zhang et al. Mol Cell Biol. 2004 Jan.
Abstract
The pathway determining malignant cellular transformation, which depends upon mutation of the BRCA1 tumor suppressor gene, is poorly defined. A growing body of evidence suggests that promotion of DNA double-strand break repair by homologous recombination (HR) may be the means by which BRCA1 maintains genomic stability, while a role of BRCA1 in error-prone nonhomologous recombination (NHR) processes has just begun to be elucidated. The BRCA1 protein becomes phosphorylated in response to DNA damage, but the effects of phosphorylation on recombinational repair are unknown. In this study, we tested the hypothesis that the BRCA1-mediated regulation of recombination requires the Chk2- and ATM-dependent phosphorylation sites. We studied Rad51-dependent HR and random chromosomal integration of linearized plasmid DNA, a subtype of NHR, which we demonstrate to be dependent on the Mre11-Rad50-Nbs1 complex. Prevention of Chk2-mediated phosphorylation via mutation of the serine 988 residue of BRCA1 disrupted both the BRCA1-dependent promotion of HR and the suppression of NHR. Similar results were obtained when endogenous Chk2 kinase activity was inhibited by expression of a dominant-negative Chk2 mutant. Surprisingly, the opposing regulation of HR and NHR did not require the ATM phosphorylation sites on serines 1423 and 1524. Together, these data suggest a functional link between recombination control and breast cancer predisposition in carriers of Chk2 and BRCA1 germ line mutations. We propose a dual regulatory role for BRCA1 in maintaining genome integrity, whereby BRCA1 phosphorylation status controls the selectivity of repair events dictated by HR and error-prone NHR.
Figures
FIG. 1.
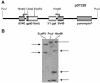
Determination of chromosomal HR in HCC1937 breast cancer cells. (A) The recombination substrate pDT220 contains two inactive copies of the bacterial gpt gene and a puromycin resistance gene. The gpt gene copies are arranged as an inverted tandem repeat (direction of transcription indicated by arrow) and are under the control of an early SV40 promoter (SV40). The upstream copy is inactivated by insertion of an I-_Sce_I recognition sequence into the unique _Kpn_I site, and the downstream copy is inactivated by a deletion of the 3′ gene region. (B) Identification of HCC1937 clones carrying a single copy of pDT220 by Southern blot analysis. Probing of the XGPRT gene after _Eco_RV digestion yielded two bands, indicating single-copy status. The _Pvu_I site was lost upon integration of the plasmid. _Hin_dIII digestion produced three bands, consistent with single-copy status, as the substrate contains two _Hin_dIII sites.
FIG. 2.
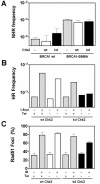
Determination of HR, radiation sensitivity, and S-phase checkpoint in BRCA1-transfected HCC1937/pDT220 cells. (A) Cells carrying a single-copy integrant of pDT220 were transfected with wild-type (wt) BRCA1, the S988A mutant, the S1423A/S1524A mutant, or an empty vector control. Pooled cell populations exhibiting similar protein expression levels in Western blot analysis were used for further study. Expression levels were compared to endogenous wild-type BRCA1 protein expression in MCF-7 breast cancer cells. (B) Spontaneous and I-_Sce_I-break-induced HR frequencies were determined for the cell populations shown in panel A. HR frequencies without I-_Sce_I expression were undetectable (<107) in cells expressing the S988A mutant. Bars with standard errors (SE) are based on the cumulative colony counts from three or four independent experiments. The relative increase of HR frequencies induced by I-_Sce_I endonuclease compared to spontaneous levels is indicated. (C) Clonogenic cell survival following exposure to IR (shown for 6-Gy data point) for cells transfected with wild-type BRCA1, the BRCA1-S988A mutant, or an empty control. Bars represent means with SE from three independent experiments. (D) Measurement of DNA synthesis 60 min following cellular exposure to 10 Gy of IR. Bars represent means with SE from three independent experiments.
FIG. 3.
Formation of Rad51 subnuclear foci in HCC1937 cells with or without exposure to IR. (A) Rad51 foci form only in a subset of cells, with and without exposure to IR. Green and blue images represent immunofluorescence labeling and DAPI nuclear DNA staining, respectively. (B) Quantitative analysis of Rad51 foci in HCC1937 cells transfected with expression vectors as described for Fig. 2, with or without exposure to 2 Gy of IR. Bars represent means with standard errors and are based on three independent experiments.
FIG. 4.
Determination of random chromosomal integration-linked NHR in NBS LBi fibroblasts and HCC1937 cells. (A) The random integration frequency of the linear plasmid substrate pcDNA3.1/hyg was determined in Nbs1-deficient cells, with or without exogenous expression of the wild-type Nbs1 protein. (B) Verification of Nbs1 protein expression using pooled populations transfected either with an empty control vector or a wild-type (wt) expression vector. (C) Verification of BRCA1 wild-type and mutant protein expression levels in single-cell-derived clones of HCC1937. (D) Plasmid integration frequency was determined in HCC1937 subclones transfected with BRCA1 expression vectors as described for Fig. 2. The logarithmic means with standard errors based on three independent experiments are shown.
FIG. 5.
Formation of Mre11 subnuclear foci in HCC1937 cells with or without exposure to IR. (A) Mre11 foci form only in a subset of cells, with and without exposure to IR. Green and blue images represent immunofluorescence labeling and DAPI nuclear DNA staining, respectively. (B) Quantitative analysis of Mre11 foci done as described for Fig. 3B except that IRIF were assessed 1 h after exposure to 2 Gy of IR. Bars represent means with standard errors and are based on three independent experiments.
FIG. 6.
Influence of Chk2 kinase activity on NHR and HR. (A) Assessment of random chromosomal integration of the pcDNA3.1/hyg substrate as described for Fig. 4. HCC1937 cells with wild-type (wt) BRCA1 or mutant BRCA1-S988A were transfected with a wt or a dominant-negative kinase-dead (kd) Chk2 expression vector in the absence of tetracycline. Bars represent the logarithmic means of three independent experiments with standard errors (SE). (B) Assessment of HR using the pDR-GFP substrate. U2OS clones 1 and 6 carry the tetracycline (Tet)-repressible expression vector for wt Ck2 and for kd Chk2, respectively. Cells were transfected with an I-_Sce_I expression vector or a control, with or without tetracycline. Bars represent the results of a comprehensive representative experiment. HR frequencies for kd Chk2 were normalized to the level of spontaneous HR in cells with tetracycline-repressed wt Chk2 expression (i.e., endogenous Chk2 only). (C) Assessment of Rad51 foci in U2OS cells with or without exposure to IR. Bars represent means with SE and are based on four independent experiments.
FIG. 7.
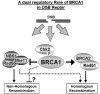
Dual-role model of BRCA1 function in regulation of recombinational repair.
Similar articles
- Ataxia telangiectasia mutated and checkpoint kinase 2 regulate BRCA1 to promote the fidelity of DNA end-joining.
Wang HC, Chou WC, Shieh SY, Shen CY. Wang HC, et al. Cancer Res. 2006 Feb 1;66(3):1391-400. doi: 10.1158/0008-5472.CAN-05-3270. Cancer Res. 2006. PMID: 16452194 - Defective Mre11-dependent activation of Chk2 by ataxia telangiectasia mutated in colorectal carcinoma cells in response to replication-dependent DNA double strand breaks.
Takemura H, Rao VA, Sordet O, Furuta T, Miao ZH, Meng L, Zhang H, Pommier Y. Takemura H, et al. J Biol Chem. 2006 Oct 13;281(41):30814-23. doi: 10.1074/jbc.M603747200. Epub 2006 Aug 10. J Biol Chem. 2006. PMID: 16905549 - Checkpoint kinase 2-mediated phosphorylation of BRCA1 regulates the fidelity of nonhomologous end-joining.
Zhuang J, Zhang J, Willers H, Wang H, Chung JH, van Gent DC, Hallahan DE, Powell SN, Xia F. Zhuang J, et al. Cancer Res. 2006 Feb 1;66(3):1401-8. doi: 10.1158/0008-5472.CAN-05-3278. Cancer Res. 2006. PMID: 16452195 - Human syndromes with genomic instability and multiprotein machines that repair DNA double-strand breaks.
De la Torre C, Pincheira J, López-Sáez JF. De la Torre C, et al. Histol Histopathol. 2003 Jan;18(1):225-43. doi: 10.14670/HH-18.225. Histol Histopathol. 2003. PMID: 12507302 Review. - The role of the BRCA1 tumor suppressor in DNA double-strand break repair.
Zhang J, Powell SN. Zhang J, et al. Mol Cancer Res. 2005 Oct;3(10):531-9. doi: 10.1158/1541-7786.MCR-05-0192. Mol Cancer Res. 2005. PMID: 16254187 Review.
Cited by
- Guarding against digestive-system cancers: Unveiling the role of Chk2 as a potential therapeutic target.
An Y, Gao D, He Y, Ge N, Guo J, Sun S, Wang C, Yang F. An Y, et al. Genes Dis. 2023 Dec 7;12(1):101191. doi: 10.1016/j.gendis.2023.101191. eCollection 2025 Jan. Genes Dis. 2023. PMID: 39524544 Free PMC article. Review. - Chemo-small extracellular vesicles released in cisplatin-resistance ovarian cancer cells are regulated by the lysosomal function.
Cerda-Troncoso C, Grünenwald F, Arias-Muñoz E, Cavieres VA, Caceres-Verschae A, Hernández S, Gaete-Ramírez B, Álvarez-Astudillo F, Acuña RA, Ostrowski M, Burgos PV, Varas-Godoy M. Cerda-Troncoso C, et al. J Extracell Biol. 2024 May 30;3(6):e157. doi: 10.1002/jex2.157. eCollection 2024 Jun. J Extracell Biol. 2024. PMID: 38947172 Free PMC article. - Well-differentiated G1 and G2 pancreatic neuroendocrine tumors: a meta-analysis of published expanded DNA sequencing data.
Andersen KØ, Detlefsen S, Brusgaard K, Christesen HT. Andersen KØ, et al. Front Endocrinol (Lausanne). 2024 May 29;15:1351624. doi: 10.3389/fendo.2024.1351624. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38868744 Free PMC article. - PARP inhibitors in prostate cancers, is it time for combinations?
Teyssonneau D, Dariane C, Barret E, Beauval JB, Brureau L, Fiard G, Fromont G, Créhange G, Gauthé M, Ruffion A, Renard-Penna R, Mathieu R, Sargos P, Rouprêt M, Ploussard G, Roubaud G. Teyssonneau D, et al. Ther Adv Med Oncol. 2024 May 31;16:17588359241242959. doi: 10.1177/17588359241242959. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 38827177 Free PMC article. Review. - Application of nitroxoline in urologic oncology - a review of evidence.
Tomczak WA, Krajewski W, Chorbińska J, Nowak Ł, Łaszkiewicz J, Grunwald K, Chełmoński A, Pisarski S, Szydełko T. Tomczak WA, et al. Contemp Oncol (Pozn). 2024;28(1):1-8. doi: 10.5114/wo.2024.139584. Epub 2024 May 19. Contemp Oncol (Pozn). 2024. PMID: 38800529 Free PMC article. Review.
References
- Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. - PubMed
- Bell, D. W., J. M. Varley, T. E. Szydlo, D. H. Kang, D. C. R. Wahrer, K. E. Shannon, M. Lubratovich, S. J. Verselis, K. J. Isselbacher, J. F. Fraumeni, J. M. Birch, F. P. Li, J. E. Garber, and D. A. Haber. 1999. Heterozygous germ line hCHK2 mutations in Li-Fraumeni syndrome. Science 286:2528-2531. - PubMed
- Bhattacharyya, A., U. S. Ear, B. H. Koller, R. R. Weichselbaum, and D. K. Bishop. 2000. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J. Biol. Chem. 275:23899-23903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous