Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation - PubMed (original) (raw)
Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation
Karen Badour et al. J Exp Med. 2004.
Abstract
Involvement of the Wiskott-Aldrich syndrome protein (WASp) in promoting cell activation requires its release from autoinhibitory structural constraints and has been attributed to WASp association with activated cdc42. Here, however, we show that T cell development and T cell receptor (TCR)-induced proliferation and actin polymerization proceed normally in WASp-/- mice expressing a WASp transgene lacking the cdc42 binding domain. By contrast, mutation of tyrosine residue Y291, identified here as the major site of TCR-induced WASp tyrosine phosphorylation, abrogated induction of WASp tyrosine phosphorylation and its effector activities, including nuclear factor of activated T cell transcriptional activity, actin polymerization, and immunological synapse formation. TCR-induced WASp tyrosine phosphorylation was also disrupted in T cells lacking Fyn, a kinase shown here to bind, colocalize with, and phosphorylate WASp. By contrast, WASp was tyrosine dephosphorylated by protein tyrosine phosphatase (PTP)-PEST, a tyrosine phosphatase shown here to interact with WASp via proline, serine, threonine phosphatase interacting protein (PSTPIP)1 binding. Although Fyn enhanced WASp-mediated Arp2/3 activation and was required for synapse formation, PTP-PEST combined with PSTPIP1 inhibited WASp-driven actin polymerization and synapse formation. These observations identify key roles for Fyn and PTP-PEST in regulating WASp and imply that inducible WASp tyrosine phosphorylation can occur independently of cdc42 binding, but unlike the cdc42 interaction, is absolutely required for WASp contributions to T cell activation.
Figures
Figure 1.
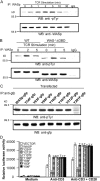
The GBD of WASp is not essential for WASp activity. (A) Scheme that shows the domain organization of wild-type and a GBD-deleted (WASpΔGBD) WASp cDNA used to derive WAS−/−ΔGBD mice. Positions of all tyrosine residues within WASp are indicated. (B) Lysates obtained from WASp or WASpΔGBD-expressing Cos-7 cells were incubated with GTPγS-loaded GST-cdc42 fusion protein bound to glutathione sepharose beads and the complexes were then resolved by SDS-PAGE and sequential immunoblotting analysis with anti-WASp and anti-cdc42 antibodies (top two panels). Immunoblotting analysis demonstrating WASp and WASpΔGBD expression in Cos-7 transfectants (Tfn) is shown in the bottom panel. (C) Immunoblotting analysis showing the WASp species detected in thymocytes and lymphocytes from wild-type (WT), WAS−/−, and WAS−/−ΔGBD mice using an anti-WASp antibody. (D) Lymphocytes and thymocytes isolated from 4–8-wk-old wild-type (WT), WAS−/−, and WAS−/−ΔGBD mice were cultured for 48 h in medium alone or with either 1 μg/ml anti-CD3 antibody or 0.2 μg/ml anti-CD3 plus anti-CD28 antibody. Antigen receptor–evoked proliferative responses were determined after an 18-h pulse with [3H]thymidine. Values represent the means (± SEM) of four independent experiments. (E) Thymocytes from wild-type (WT), WAS−/−, and WAS−/−ΔGBD mice were isolated and stimulated with anti-CD3 plus anti-CD28 antibodies for 30 min on ice followed by cross-linking with a secondary antibody. Cells were fixed with 5% paraformaldehyde and F-actin content was quantified by flow cytometric analysis of FITC phalloidin–stained resting (bold lines) and stimulated (dashed lines) cells. The results are representative of three independent experiments.
Figure 2.
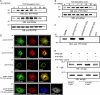
TCR-induced phosphorylation of WASp Y291 is required for induction of NFAT activity, actin polymerization, and immunological synapse formation. (A) Lysates prepared from resting or TCR-stimulated Jurkat T cells at the indicated times after stimulation were immunoprecipitated with anti-WASp antibody or control IgG subjected to SDS-PAGE and sequential immunoblotting analysis with anti-pTyr and anti-WASp antibodies. (B) Lysates prepared from wild-type (WT) or WAS−/−ΔGBD thymocytes at the indicated times after stimulation were immunoprecipitated with anti-WASp antibody or control IgG and the complexes were then subjected to SDS-PAGE and sequential immunoblotting analysis with anti-pTyr and anti-WASp antibodies. (C) Jurkat cells were transfected with pEGFP vector containing either the wild-type (WT) WASp cDNA or WASp cDNAs carrying each of the indicated Y→F substitutions. Lysates prepared from the TCR-stimulated cells were immunoprecipitated with anti-GFP antibody and the immunoprecipitated proteins were then subjected to SDS-PAGE and sequential immunoblotting analysis with anti-pTyr and anti-GFP antibodies. (D) Thymocytes from WAS−/− mice were cotransfected with pEGFP vectors containing WASp or the indicated WASp tyrosine phenylalanine (Y→F) mutant cDNAs and an NFAT luciferase reporter vector. At 4 h after transfection, cells were stimulated with anti-CD3 and anti-CD28 antibodies. After 8 h of incubation, cells were lysed and assayed by luminometry for luciferase expression. Values represent the means (± SEM) of three assays and the results are representative of four independent experiments. (E) Thymocytes from WAS−/− mice were transfected with pEGFP-WASp, WASpY291F, WASpY212F, or WASpY88F and the cells were either left unstimulated or stimulated with anti-CD3 and anti-CD28 antibodies for 30 min on ice followed by cross-linking with anti–hamster Ig secondary antibody. Cells were fixed with 5% paraformaldehyde and F-actin content was quantified by flow cytometric analysis of FITC phalloidin–stained cells. The results are representative of three independent experiments. (F) Lymphocytes from WAS−/−/OT-II mice were either untreated (a) or transfected with pEGFP-WASp (b), pEGFP-WASpY102F (c), or pEGFP-WASpY291F (d) and the cells were then incubated with OVA329–339–pulsed LB27.4 cells, fixed, and stained for actin and PKC-θ, and then visualized by immunofluorescent microscopy. The images on the far right of each panel represent merges of the other three images within the panel. Data shown are representative of four independent experiments.
Figure 2.
TCR-induced phosphorylation of WASp Y291 is required for induction of NFAT activity, actin polymerization, and immunological synapse formation. (A) Lysates prepared from resting or TCR-stimulated Jurkat T cells at the indicated times after stimulation were immunoprecipitated with anti-WASp antibody or control IgG subjected to SDS-PAGE and sequential immunoblotting analysis with anti-pTyr and anti-WASp antibodies. (B) Lysates prepared from wild-type (WT) or WAS−/−ΔGBD thymocytes at the indicated times after stimulation were immunoprecipitated with anti-WASp antibody or control IgG and the complexes were then subjected to SDS-PAGE and sequential immunoblotting analysis with anti-pTyr and anti-WASp antibodies. (C) Jurkat cells were transfected with pEGFP vector containing either the wild-type (WT) WASp cDNA or WASp cDNAs carrying each of the indicated Y→F substitutions. Lysates prepared from the TCR-stimulated cells were immunoprecipitated with anti-GFP antibody and the immunoprecipitated proteins were then subjected to SDS-PAGE and sequential immunoblotting analysis with anti-pTyr and anti-GFP antibodies. (D) Thymocytes from WAS−/− mice were cotransfected with pEGFP vectors containing WASp or the indicated WASp tyrosine phenylalanine (Y→F) mutant cDNAs and an NFAT luciferase reporter vector. At 4 h after transfection, cells were stimulated with anti-CD3 and anti-CD28 antibodies. After 8 h of incubation, cells were lysed and assayed by luminometry for luciferase expression. Values represent the means (± SEM) of three assays and the results are representative of four independent experiments. (E) Thymocytes from WAS−/− mice were transfected with pEGFP-WASp, WASpY291F, WASpY212F, or WASpY88F and the cells were either left unstimulated or stimulated with anti-CD3 and anti-CD28 antibodies for 30 min on ice followed by cross-linking with anti–hamster Ig secondary antibody. Cells were fixed with 5% paraformaldehyde and F-actin content was quantified by flow cytometric analysis of FITC phalloidin–stained cells. The results are representative of three independent experiments. (F) Lymphocytes from WAS−/−/OT-II mice were either untreated (a) or transfected with pEGFP-WASp (b), pEGFP-WASpY102F (c), or pEGFP-WASpY291F (d) and the cells were then incubated with OVA329–339–pulsed LB27.4 cells, fixed, and stained for actin and PKC-θ, and then visualized by immunofluorescent microscopy. The images on the far right of each panel represent merges of the other three images within the panel. Data shown are representative of four independent experiments.
Figure 3.
Fyn binds and phosphorylates WASp after TCR engagement. (A) Lymphocytes from Itk−/−, Fyn−/−, and wild-type mice as well as JCam-1 (Lck-deficient) and Lck-reconstituted JCam-1 (JCam + Lck) cells were stimulated with anti-CD3/anti-CD28 antibodies and cell lysates were then prepared and immunoprecipitated with anti-WASp antibody. Complexes were subjected to SDS-PAGE and sequential immunoblotting analysis with anti-pTyr and anti-WASp antibodies. (B) Lysates were prepared from anti-CD3/anti-CD28–stimulated Jurkat cell transfectants expressing cDNAs for wild-type Fyn and FynK296M alone or in combination. After immunoprecipitation with anti-WASp antibody, complexes were subjected to immunoblotting analysis using anti-pTyr and then anti-WASp antibodies (top two panels). Fyn expression levels in the transfected cells lysates are shown in the bottom panel. (C) Lysates were prepared from unstimulated (U) or stimulated (S) Jurkat cells cotransfected with Fyn, WASp, and one of either the cdc42 wild-type (WT), the cdc42-V12, or N17 mutant cDNAs. Lysate proteins were immunoprecipitated with anti-WASp antibody and subjected to SDS-PAGE and sequential immunoblotting with anti-pTyr and anti-WASp antibodies (top two panels). Expression of Fyn and cdc42 in the lysates is shown in the bottom two panels. (D) Jurkat cells were stimulated for the indicated time periods with anti-CD3 and anti-CD28 antibodies, lysed, and the lysate proteins were immunoprecipitated with anti-WASp antibody. Complexes, lysate proteins, and IgG were subjected to SDS-PAGE and sequential immunoblotting analysis with anti-Fyn and anti-WASp antibodies. (E) Purified His-tagged Fyn fusion protein was incubated with GST-WASp, GST-WASpΔPro, or GST fusion proteins bound to glutathione sepharose beads. The complexes were washed and subjected to SDS-PAGE and sequential immunoblotting with anti-Fyn and anti-GST antibodies. (F) Jurkat cells were stimulated, lysed, and the lysate proteins were subjected to anti-Fyn antibody immunoprecipitation. Immunoprecipitates were then incubated in kinase buffer containing 10 μg [γ-32P] ATP and either GST, GST-WASp, GST-WASpΔPro, or GST-WASp Y291F fusion proteins bound to glutathione sepharose beads. Complexes were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and phosphorylation was analyzed by autoradiography. The position of phosphorylated GST-WASp is indicated. This result is representative of four independent assays.
Figure 4.
WASp inducibly associates and colocalizes with PSTPIP1 and PTP-PEST. (A) Jurkat T cells were stimulated for the indicated times with anti-CD3 and anti-CD28 antibodies and lysates were then prepared and immunoprecipitated with anti-PSTPIP1 antibody. The immune complexes were subjected to SDS-PAGE and sequentially immunoblotted with anti-PST-PEST, anti-WASp, and anti-PSTPIP1 antibodies. (B) Jurkat T cells were stimulated with anti-CD3 and anti-CD28 antibodies and lysates were then prepared and immunoprecipitated with anti-WASp antibody. Complexes were resolved by SDS-PAGE followed by sequential immunoblotting with anti–PTP-PEST and anti-WASp antibodies. (C) Lysates prepared from Jurkat T cells were incubated with GST, GST-PSTPIP1, full-length (FL), GST-PSTPIPCOIL, or GST-PSTPIPSH3 fusion proteins bound to glutathione- sepharose beads. Complexes were resolved by SDS-PAGE and immunoblotted using an anti–PTP-PEST antibody. (D) Cos-7 cells were transiently transfected with pEGFP-PSTPIP1 (a), pEGFP-PTP-PEST (b), pEGFP-WASp (c), pEGFP-PSTPIP1 and pcΔNA3-PTP-PEST (d), or pEGFP-PSTPIP1, DSRED-WASp, and pcDNA3-PTP-PEST (e). Cells were fixed, stained with rhodamine phalloidin for actin (a–c) or with anti–PTP-PEST antibody (d and e) and Cy5 anti–rabbit Ig (e), and then analyzed by confocal immunofluorescent microscopy. The images shown are representative of three independent experiments. (E) pcDNA3 constructs for expression of wild-type or catalytically inactive (C231S) PTP-PEST were cotransfected with pEGFP-WASp (WASp-GFP) into Jurkat cells. The cells were either left unstimulated or stimulated with anti-CD3 and anti-CD28 antibodies, lysed, and the lysate proteins were immunoprecipitated with anti-GFP antibodies. The complexes were subjected to SDS-PAGE and immunoblotted sequentially with anti–p-Tyr and anti-GFP antibodies.
Figure 5.
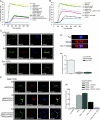
Fyn and PTP-PEST modulate WASp effects on induction of actin polymerization and synapse formation. (A) Polymerization of 2.8 μM pyrene-labeled actin monomer was assayed in the presence of 20 nM Arp2/3 complex, 100 nM GST-WASp or GST-WASpΔGBD, and GST fusion proteins containing 50–500 nM either Fyn, PTP-PEST, PSTPIP, or cdc42-V12. Polymerization was monitored by the increase in prenyl-actin fluorescence. B. The pyrene actin assay was used to compare the WASp-Arp2/3–actin polymerizing activities of cdc42 at low (15 nM), medium (250 nM), or high (500 nM) concentration and Fyn at low (10 nM), medium (100 nM), or high (200 nM) concentration alone or in combination. (C) Fyn effects on synapse formation were evaluated by incubating lymphocytes from OT-II (a and b) and Fyn−/−/OT-II (c) mice with unpulsed (a) or OVA peptide–pulsed (b and c) LB27.4 cells followed by cell fixation, staining for WASp, Fyn, and actin, and visualization by immunofluorescent microscopy. Images on the far left of each panel represent a merge of the other three images within each panel. A computer-generated three-dimensional reconstruction of the synaptic region formed between wild-type T cells and APCs (d) shows the localization of Fyn in the central area of the synapse and the distribution of WASp in both the central and peripheral synaptic region. Synapses were quantified (e) by counting the numbers of T cell–APC conjugates showing clustered actin at the conjugation site. Values shown are the percent of conjugates with synapse formation and represent means (± SEM) of three independent experiments. (D) PTP-PEST effects on synapse formation were assessed using WAS−/−/OT-II lymphocytes transfected with pDSRED-WASp (a), pcDNA3-WASp and pDSRED-PSTPIP1 (b), or pcDNA3-WASp, pEGFP-PSTPIP1, and pDSRED-PTP-PEST (c). Cells were incubated with OVA peptide–pulsed LB27.4 B cells, fixed, and stained for actin and/or PKC-θ, and visualized by immunofluorescent microscopy. The image on the far right of each panel is a merged image of all other images in the panel. Synapses were quantified by counting the number of T cell–B cell conjugates showing clustered actin at the synaptic site. Values shown are the percent of conjugates with synapse formation and represent the means (± SEM) of three independent experiments.
Similar articles
- Identification of Fyn as the binding partner for the WASP N-terminal domain in T cells.
Sato M, Sawahata R, Takenouchi T, Kitani H. Sato M, et al. Int Immunol. 2011 Aug;23(8):493-502. doi: 10.1093/intimm/dxr042. Epub 2011 Jun 24. Int Immunol. 2011. PMID: 21705469 - SLP-76 coordinates Nck-dependent Wiskott-Aldrich syndrome protein recruitment with Vav-1/Cdc42-dependent Wiskott-Aldrich syndrome protein activation at the T cell-APC contact site.
Zeng R, Cannon JL, Abraham RT, Way M, Billadeau DD, Bubeck-Wardenberg J, Burkhardt JK. Zeng R, et al. J Immunol. 2003 Aug 1;171(3):1360-8. doi: 10.4049/jimmunol.171.3.1360. J Immunol. 2003. PMID: 12874226 - Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes.
Zhang J, Shehabeldin A, da Cruz LA, Butler J, Somani AK, McGavin M, Kozieradzki I, dos Santos AO, Nagy A, Grinstein S, Penninger JM, Siminovitch KA. Zhang J, et al. J Exp Med. 1999 Nov 1;190(9):1329-42. doi: 10.1084/jem.190.9.1329. J Exp Med. 1999. PMID: 10544204 Free PMC article. - Wiskott-Aldrich syndrome protein--dynamic regulation of actin homeostasis: from activation through function and signal termination in T lymphocytes.
Matalon O, Reicher B, Barda-Saad M. Matalon O, et al. Immunol Rev. 2013 Nov;256(1):10-29. doi: 10.1111/imr.12112. Immunol Rev. 2013. PMID: 24117810 Review. - WASP (Wiskott-Aldrich syndrome protein) gene mutations and phenotype.
Imai K, Nonoyama S, Ochs HD. Imai K, et al. Curr Opin Allergy Clin Immunol. 2003 Dec;3(6):427-36. doi: 10.1097/00130832-200312000-00003. Curr Opin Allergy Clin Immunol. 2003. PMID: 14612666 Review.
Cited by
- Molecular mechanisms of phenotypic variability in monogenic autoinflammatory diseases.
Aksentijevich I, Schnappauf O. Aksentijevich I, et al. Nat Rev Rheumatol. 2021 Jul;17(7):405-425. doi: 10.1038/s41584-021-00614-1. Epub 2021 May 25. Nat Rev Rheumatol. 2021. PMID: 34035534 Review. - Protein tyrosine phosphatase function: the substrate perspective.
Tiganis T, Bennett AM. Tiganis T, et al. Biochem J. 2007 Feb 15;402(1):1-15. doi: 10.1042/BJ20061548. Biochem J. 2007. PMID: 17238862 Free PMC article. Review. - Impaired podosome formation and invasive migration of macrophages from patients with a PSTPIP1 mutation and PAPA syndrome.
Cortesio CL, Wernimont SA, Kastner DL, Cooper KM, Huttenlocher A. Cortesio CL, et al. Arthritis Rheum. 2010 Aug;62(8):2556-8. doi: 10.1002/art.27521. Arthritis Rheum. 2010. PMID: 20506269 Free PMC article. No abstract available. - Diverse levels of sequence selectivity and catalytic efficiency of protein-tyrosine phosphatases.
Selner NG, Luechapanichkul R, Chen X, Neel BG, Zhang ZY, Knapp S, Bell CE, Pei D. Selner NG, et al. Biochemistry. 2014 Jan 21;53(2):397-412. doi: 10.1021/bi401223r. Epub 2014 Jan 7. Biochemistry. 2014. PMID: 24359314 Free PMC article. - Immune-mediated inflammatory diseases with chronic excess of serum interleukin-18.
Miyazawa H, Wada T. Miyazawa H, et al. Front Immunol. 2022 Jul 25;13:930141. doi: 10.3389/fimmu.2022.930141. eCollection 2022. Front Immunol. 2022. PMID: 35958573 Free PMC article. Review.
References
- Badour, K., J. Zhang, and K.A. Siminovitch. 2003. The Wiskott-Aldrich syndrome protein: forging a link between actin and cell activation. Immunol. Rev. 192:98–112. - PubMed
- Machesky, L.M., and R.H. Insall. 1998. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8:1347–1356. - PubMed
- Rohatgi, R., L. Ma, H. Miki, M. Lopez, T. Kirchhausen, T. Takenawa, and M.W. Kirschner. 1999. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 97:221–231. - PubMed
- Cory, G.O., R. Cramer, L. Blanchoin, and A.J. Ridley. 2003. Phosphorylation of the WASp-VCA domain increases its affinity for the Arp2/3 complex and enhances actin polymerization by WASp. Mol. Cell. 11:1229–1239. - PubMed
- Badour, K., J. Zhang, F. Shi, M.K. McGavin, V. Ramperasad, L.A. Hardy, D. Field, and K.A. Siminovitch. 2003. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity. 18:141–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous