Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris - PubMed (original) (raw)
Comparative Study
Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris
Edmund Lee et al. J Exp Med. 2004.
Abstract
Psoriasis is a type I-deviated disease characterized by the presence of interferon (IFN)-gamma and multiple IFN-related inflammatory genes in lesions. Because interleukin (IL)-23 is now recognized to play a role in the recruitment of inflammatory cells in a T helper cell (Th)1-mediated disease, we examined psoriasis skin lesions for production of this newly described cytokine. IL-23 is composed of two subunits: a unique p19 subunit and a p40 subunit shared with IL-12. We found a reliable increase in p19 mRNA by quantitative reverse transcription polymerase chain reaction in lesional skin compared with nonlesional skin (22.3-fold increase; P = 0.001). The p40 subunit, shared by IL-12 and IL-23, increased by 11.6-fold compared with nonlesional skin (P = 0.003), but the IL-12 p35 subunit was not increased in lesional skin. IL-23 was expressed mainly by dermal cells and increased p40 immunoreactivity was visualized in large dermal cells in the lesions. Cell isolation experiments from psoriatic tissue showed strong expression of p19 mRNA in cells expressing monocyte (CD14+ CD11c+ CD83-) and mature dendritic cell (DC) markers (CD14- CD11c+ CD83+), whereas in culture, the mRNAs for p40 and p19 were strongly up-regulated in stimulated monocytes and monocyte-derived DCs, persisting in the latter for much longer periods than IL-12. Our data suggest that IL-23 is playing a more dominant role than IL-12 in psoriasis, a Th1 type of human inflammatory disease.
Figures
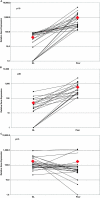
Figure 1.
Expression of p19, p40, and p35 mRNA in paired samples of uninvolved and lesional skin from psoriasis patients. ♦, indicates the arithmetic mean for each type of specimen and is not connected by a line. A line connects the paired samples taken from each patient's nonlesional and psoriatic skin. Data is presented as the ratio of PCR yield, in picograms, of p19 (A), p40 (B), and p35 (C) normalized to the yield of hARP. Note the logarithmic scale of the abscissa.
Figure 2.
Immunohistochemical localization of p40 in psoriatic skin. The four panels are from frozen sections of human skin from psoriasis patients. A and B are IgG isotype controls in uninvolved and lesional skin, respectively. C and D are stained with mouse anti–human IL-12p40 as indicated. The arrow indicates morphology consistent with DCs. The color represents 3-amino-9-ethylcarbazole deposition at the site of antibody binding. Melanin appears in A as dark stain in the basal cell layer.
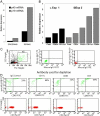
Figure 3.
p19 and p40 gene expression in dispase split skin and dermally derived inflammatory cells. (A) Dermis from psoriasis lesions has greater p19 and p40 mRNA than epidermis. The data are from one representative experiment. A repeat run produced almost identical results. RNA was isolated from dispase split lesional skin taken from psoriatic plaques from two patients. RT-PCR for the p19 and p40 genes was performed on the epidermis and dermis and gene expression levels are shown relative to the hARP gene. In two other patients, the dermis was cultured for an additional 2 d at 37°C and the cells exiting the cultured dermis (B, C, and D) were collected for subsequent quantitative gene expression for p19 using immunomagnetic beads coated with antibodies to CD3, CD11c, or CD83. In each case, two cell populations were obtained: one adherent to the beads expressing CD83, CD11c, or CD68 (B), and one nonadherent (C and D) that was used to monitor the depletion by FACS®. For the latter, flow-through cells were analyzed for the presence of HLA-DR and CD83. (B) Total RNA was isolated and gene expression for p19 was assayed using TaqMan chemistry as described in Materials and Methods. Data are from one representative experiment. A repeat run produced almost identical results. (C, left) Side and forward scatter plots and gating (R1 for lymphocytes and R2 for DCs) for subsequent panels. (C, middle and C, right) Isotype controls. Cells depleted by isotype control (D, top, panel 1), anti-CD11c (D, top, panel 2), anti-CD83 (D, top, panel 3), and anti-CD3 (D, top, panel 4). Note that percentages represent upper right quadrant cell count (HLA-DR+ CD83+) as percent of total. Each panel has a plot of forward scatter versus CD3+ to demonstrate additional specificity of the depletion.
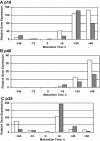
Figure 4.
Expression of p19, p40, and p35 mRNA in monocyte-derived DCs. Monocytes were placed in culture at −144 h, differentiated to immature DCs, and then stimulated beginning at 0 h with TNF-α, IL-1β, IL-6, and PGE2 for the stated amount of time. The data bars (open and solid) represent relative gene expression of p19 (A), p40 (B), and p35 (C) from two separate experiments. Note differing abscissa scales.
Similar articles
- In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin.
Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. Piskin G, et al. J Immunol. 2006 Feb 1;176(3):1908-15. doi: 10.4049/jimmunol.176.3.1908. J Immunol. 2006. PMID: 16424222 - The expression of interleukin-23 (p19/p40) and interleukin-12 (p35/p40) in psoriasis skin.
Chen X, Tan Z, Yue Q, Liu H, Liu Z, Li J. Chen X, et al. J Huazhong Univ Sci Technolog Med Sci. 2006;26(6):750-2. doi: 10.1007/s11596-006-0635-z. J Huazhong Univ Sci Technolog Med Sci. 2006. PMID: 17357509 - Increased expression of IL-12p70 and IL-23 by multiple dendritic cell and macrophage subsets in plaque psoriasis.
Yawalkar N, Tscharner GG, Hunger RE, Hassan AS. Yawalkar N, et al. J Dermatol Sci. 2009 May;54(2):99-105. doi: 10.1016/j.jdermsci.2009.01.003. Epub 2009 Mar 4. J Dermatol Sci. 2009. PMID: 19264456 - Interleukin-12, interleukin-23, and psoriasis: current prospects.
Torti DC, Feldman SR. Torti DC, et al. J Am Acad Dermatol. 2007 Dec;57(6):1059-68. doi: 10.1016/j.jaad.2007.07.016. Epub 2007 Aug 15. J Am Acad Dermatol. 2007. PMID: 17706835 Review. - Interleukin-23: a key cytokine in inflammatory diseases.
Duvallet E, Semerano L, Assier E, Falgarone G, Boissier MC. Duvallet E, et al. Ann Med. 2011 Nov;43(7):503-11. doi: 10.3109/07853890.2011.577093. Epub 2011 May 17. Ann Med. 2011. PMID: 21585245 Review.
Cited by
- The roles of T cells in psoriasis.
Zhang P, Su Y, Li S, Chen H, Wu R, Wu H. Zhang P, et al. Front Immunol. 2023 Oct 24;14:1081256. doi: 10.3389/fimmu.2023.1081256. eCollection 2023. Front Immunol. 2023. PMID: 37942312 Free PMC article. Review. - Current perspective on the role of the interleukin-23/interleukin-17 axis in inflammation and disease (chronic arthritis and psoriasis).
Cauli A, Piga M, Floris A, Mathieu A. Cauli A, et al. Immunotargets Ther. 2015 Oct 1;4:185-90. doi: 10.2147/ITT.S62870. eCollection 2015. Immunotargets Ther. 2015. PMID: 27471723 Free PMC article. Review. - Nonclinical evaluations of deucravacitinib and Janus kinase inhibitors in homeostatic and inflammatory pathways.
Johnson B, Cheng L, Koenitzer J, Catlett IM, Schafer P. Johnson B, et al. Front Immunol. 2024 Sep 30;15:1437512. doi: 10.3389/fimmu.2024.1437512. eCollection 2024. Front Immunol. 2024. PMID: 39403378 Free PMC article. - The Role of the IL-23/IL-17 Pathway in the Pathogenesis of Spondyloarthritis.
Tsukazaki H, Kaito T. Tsukazaki H, et al. Int J Mol Sci. 2020 Sep 3;21(17):6401. doi: 10.3390/ijms21176401. Int J Mol Sci. 2020. PMID: 32899140 Free PMC article. Review.
References
- Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. - PubMed
- Parham, C., M. Chirica, J. Timans, E. Vaisberg, M. Travis, J. Cheung, S. Pflanz, R. Zhang, K.P. Singh, F. Vega, et al. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699–5708. - PubMed
- Wiekowski, M.T., M.W. Leach, E.W. Evans, L. Sullivan, S.C. Chen, G. Vassileva, J.F. Bazan, D.M. Gorman, R.A. Kastelein, S. Narula, et al. 2001. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J. Immunol. 166:7563–7570. - PubMed
- Cua, D.J., J. Sherlock, Y. Chen, C.A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1K23 AR 49815-01/AR/NIAMS NIH HHS/United States
- R01 AI049572/AI/NIAID NIH HHS/United States
- M01 RR000102/RR/NCRR NIH HHS/United States
- M01 RR 00102/RR/NCRR NIH HHS/United States
- R01 AI049832/AI/NIAID NIH HHS/United States
- AI 49832/AI/NIAID NIH HHS/United States
- P01 CA084512/CA/NCI NIH HHS/United States
- K23 AR049815/AR/NIAMS NIH HHS/United States
- AI 49572/AI/NIAID NIH HHS/United States
- CA 84512/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials