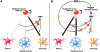IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes - PubMed (original) (raw)
IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes
Jenny Hsieh et al. J Cell Biol. 2004.
Abstract
Adult multipotent neural progenitor cells can differentiate into neurons, astrocytes, and oligodendrocytes in the mammalian central nervous system, but the molecular mechanisms that control their differentiation are not yet well understood. Insulin-like growth factor I (IGF-I) can promote the differentiation of cells already committed to an oligodendroglial lineage during development. However, it is unclear whether IGF-I affects multipotent neural progenitor cells. Here, we show that IGF-I stimulates the differentiation of multipotent adult rat hippocampus-derived neural progenitor cells into oligodendrocytes. Modeling analysis indicates that the actions of IGF-I are instructive. Oligodendrocyte differentiation by IGF-I appears to be mediated through an inhibition of bone morphogenetic protein signaling. Furthermore, overexpression of IGF-I in the hippocampus leads to an increase in oligodendrocyte markers. These data demonstrate the existence of a single molecule, IGF-I, that can influence the fate choice of multipotent adult neural progenitor cells to an oligodendroglial lineage.
Figures
Figure 1.
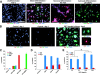
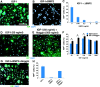
IGF-I–induced differentiation of multipotent neural progenitor cells. (A) Adult hippocampus-derived neural progenitor cells cultured in insulin-containing N2 media with 20 ng/ml FGF-2 (undifferentiated), 1 μM RA, and 1% FBS for 4 d (mixed), 1 μM RA and 5 μM forskolin for 4 d (neuronal), or 50 ng/ml leukemia inhibitory factor and 50 ng/ml BMP2 for 6 d (astrocytic). Cells were stained for markers for neurons (Tuj1), astrocytes (GFAP), or oligodendrocytes (RIP), and also with DAPI. Bar, 50 μm. (B) Differentiation of neural progenitor cells in insulin-free N2 media without IGF-I (control) or with IGF-I for 4 d. Insets show cells of typical oligodendrocyte morphology stained with RIP or MBP. Bar, 25 μm. (C) Quantification of cells in either proliferating or differentiating conditions. (D) Quantification of cultures grown in insulin-free N2 media (control) or treated with 500 ng/ml IGF-I, IGF-II, or insulin after 4 d. (E) Quantification of cells in response to different doses of IGF-I (20–500 ng/ml) in 4-d cultures. All data shown are from at least three experiments in parallel cultures with error bars representing SDs. Significant differences are indicated with an asterisk (P < 0.005).
Figure 2.
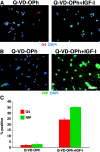
IGF-I–mediated increase in oligodendrocyte differentiation is independent of effects on neural progenitor cell survival in 2-d cultures. (A) Treatment of cells with 2 μM Q-VD-OPh resulted in a general absence of apoptosis in short-term cultures. The maintenance of cell survival is shown by a general persistence of cells and a lack of fragmented DAPI-stained nuclei, without obvious effects on cell proliferation or differentiation. Addition of 500 ng/ml IGF-I to the Q-VD-OPh–treated cultures mediated an increase in oligodendrocyte differentiation, as evidenced by O4 staining and morphological criteria. (B) Similar results were seen with RIP. (C) Quantification of O4 and RIP+ oligodendrocytes. Bar, 25 μm.
Figure 3.
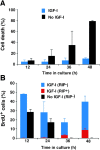
Effect of IGF-I on the survival, proliferation, and instructive oligodendrocyte differentiation of adult neural progenitor cells. (A) Quantification of cell death in insulin-free (no IGF-I) and IGF-I–treated (500 ng/ml) cultures, as determined every 12 h by live staining with 1 μg/ml propidium iodide and 1 μg/ml Hoechst 33342. (B) Proliferation in insulin-free (no IGF-I) and IGF-I–treated (500 ng/ml) cultures as determined by BrdU incorporation. Parallel cultures were incubated with 2.5 μM BrdU at the beginning of each 12-h time point, immediately followed by fixation and BrdU staining. BrdU+ cells were counted as either being RIP− (blue and black columns) or RIP+ (red columns). All data are from at least three independent experiments in parallel cultures with error bars representing SDs.
Figure 4.
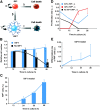
IGF-I instructs neural progenitor cells to commit to an oligodendroglial lineage and increases the proliferation of committed oligodendrocytes. (A) Adult neural progenitor cell states in culture: α, proliferation rate of progenitors (RIP−); β, differentiation rate of progenitors (RIP−) to oligodendrocytes (RIP+); δ, cell death rate for progenitors (RIP−); γ, proliferation rate for oligodendrocytes (RIP+); ɛ, cell death rate for oligodendrocytes (RIP+). (B and C) Comparison between direct measurements (columns) and modeling analyses (lines) of the total cell numbers (B) and of the percentage of RIP+ cells (C). Black columns are insulin-free (no IGF-I) conditions, and blue columns are 500 ng/ml IGF-I conditions. No significant differences were found between the direct measurements and the values predicted by the model in each case. All data were normalized to values at the 12-h time point within each condition due to variability in cell behavior at time 0 (4 h after plating). (D) The proliferation rates for RIP− (α) and RIP+ (γ) cells in insulin-free (no IGF-I) conditions (black line) and treated with 500 ng/ml IGF-I (blue and red lines). (E) The differentiation rate for RIP− (β) cells treated with 500 ng/ml IGF-I. These data are representative of at least three independent experiments. Error bars (except in D and E) represent SDs. The error bars in D and E represent the upper and lower bounds for proliferation (α, γ) and differentiation (β) rates.
Figure 5.
IGF-I–mediated oligodendrocyte differentiation involves an inhibition of BMP signaling. (A and B) Cells treated with 500 ng/ml IGF-I alone (A) or in combination with 50 ng/ml BMP2 (B). Cells were stained for an oligodendrocyte marker (RIP) and DAPI. (C) Quantification of RIP+ or cells treated with 500 ng/ml IGF-I alone or with different doses of BMP2 (0.05–50 ng/ml) in 4-d cultures. (D and E) Cells treated with 50 ng/ml IGF-I alone or in combination with 500 ng/ml Noggin. (F) Quantification of cells treated with different doses of IGF-I (20–1,000 ng/ml) alone (blue columns) or in conjunction with 500 ng/ml Noggin (black columns). Significant differences are indicated with an asterisk (P < 0.05, t test). (G) Cells treated with a combination of IGF-I, BMP2, and Noggin. (H) Quantification of RIP+ cells in IGF-I alone, IGF-I + BMP2, and IGF-I + BMP2 + Noggin. For G and H, concentrations are as follows: IGF-I, 500 ng/ml; BMP2, 50 ng/ml; Noggin, 500 ng/ml. Bar, 50 μm. All error bars represent SDs.
Figure 6.
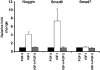
IGF-I induction of oligodendrocyte differentiation is associated with an up-regulation of BMP antagonists Noggin, Smad6, and Smad7. Q-PCR analysis and quantification of relative fold change (normalized to expression levels under FGF-2 conditions) of Noggin, Smad6, and Smad7. RNA was harvested from 24-h cultures treated with 20 ng/ml FGF-2, 500 ng/ml IGF-I, or both. All error bars represent SDs.
Figure 7.
IGF-I overexpression in the hilus promotes oligodendrocyte differentiation in vivo. (A–J) Representative images of brain sections focusing in on the hilar region in animals injected with rAAV-β-gal controls (A–D and I) and rAAV-IGF-I (E–H and J). Sections were triple labeled with antibodies to oligodendrocyte markers RIP (A and E) and MBP (B and F), and an astrocyte marker GFAP (C and G). Merged images are shown in D and H; RIP is in red, MBP is in blue, GFAP is in green. (I and J) Representative sections stained with the oligodendrocyte marker GST-π in red and DAPI to visualize cell nuclei. White arrows indicate cells that are GST-π–positive. (K) The average number of cells in the hilus (in three adjacent fields distal to the injection site) per section in which GST-π was detected in each animal group (rAAV-β-gal animals, n = 3; rAAV-IGF-I animals, n = 4) is plotted. The asterisk indicates that values are significantly different between control and IGF-I–overexpressed animals (P < 0.001, t test), and error bars represent SDs. Bar, 100 μm.
Figure 8.
Proposed model for the role of IGFs in multipotent neural progenitor cell fate specification: oligodendroglial and neuronal fate commitment at the expense of astroglial fates? (A) BMP signaling has been shown to stimulate astroglial differentiation and inhibit neuronal and oligodendroglial differentiation. (B) Activation of the IGF-I receptor on multipotent neural progenitor cells by IGFs leads to the up-regulation of Noggin, Smad6, and Smad7. Because Noggin, Smad6, and Smad7 inhibit BMP signaling, the net effects of IGF signaling are a block in astrocyte differentiation and an induction of neuronal and oligodendroglial differentiation. Alternatively, IGF-instructive effects on oligodendrocyte differentiation could occur in a Noggin/Smad6, Smad7-independent pathway.
Similar articles
- Large-scale generation of highly enriched neural stem-cell-derived oligodendroglial cultures: maturation-dependent differences in insulin-like growth factor-mediated signal transduction.
Broughton SK, Chen H, Riddle A, Kuhn SE, Nagalla S, Roberts CT Jr, Back SA. Broughton SK, et al. J Neurochem. 2007 Feb;100(3):628-38. doi: 10.1111/j.1471-4159.2006.04171.x. J Neurochem. 2007. PMID: 17263792 - Potential of progenitors from postnatal cerebellar neuroepithelium and white matter: lineage specified vs. multipotent fate.
Milosevic A, Goldman JE. Milosevic A, et al. Mol Cell Neurosci. 2004 Jun;26(2):342-53. doi: 10.1016/j.mcn.2004.02.008. Mol Cell Neurosci. 2004. PMID: 15207858 - A population of oligodendrocytes derived from multipotent neural precursor cells expresses a cholinergic phenotype in culture and responds to ciliary neurotrophic factor.
MacDonald SC, Simcoff R, Jordan LM, Dodd JG, Cheng KW, Hochman S. MacDonald SC, et al. J Neurosci Res. 2002 May 1;68(3):255-64. doi: 10.1002/jnr.10200. J Neurosci Res. 2002. PMID: 12111855 - Postnatal cerebral cortical multipotent progenitors: regulatory mechanisms and potential role in the development of novel neural regenerative strategies.
Mehler MF, Gokhan S. Mehler MF, et al. Brain Pathol. 1999 Jul;9(3):515-26. doi: 10.1111/j.1750-3639.1999.tb00539.x. Brain Pathol. 1999. PMID: 10416991 Free PMC article. Review. - The early steps of oligodendrogenesis: insights from the study of the plp lineage in the brain of chicks and rodents.
Spassky N, Olivier C, Cobos I, LeBras B, Goujet-Zalc C, Martínez S, Zalc B, Thomas JL. Spassky N, et al. Dev Neurosci. 2001;23(4-5):318-26. doi: 10.1159/000048715. Dev Neurosci. 2001. PMID: 11756747 Review.
Cited by
- Characterization of Functionally Associated miRNAs in Glioblastoma and their Engineering into Artificial Clusters for Gene Therapy.
Bhaskaran V, Peruzzi P. Bhaskaran V, et al. J Vis Exp. 2019 Oct 4;(152):10.3791/60215. doi: 10.3791/60215. J Vis Exp. 2019. PMID: 31633695 Free PMC article. - Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development.
Montgomery RL, Hsieh J, Barbosa AC, Richardson JA, Olson EN. Montgomery RL, et al. Proc Natl Acad Sci U S A. 2009 May 12;106(19):7876-81. doi: 10.1073/pnas.0902750106. Epub 2009 Apr 20. Proc Natl Acad Sci U S A. 2009. PMID: 19380719 Free PMC article. - The role of adult hippocampal neurogenesis in brain health and disease.
Toda T, Parylak SL, Linker SB, Gage FH. Toda T, et al. Mol Psychiatry. 2019 Jan;24(1):67-87. doi: 10.1038/s41380-018-0036-2. Epub 2018 Apr 20. Mol Psychiatry. 2019. PMID: 29679070 Free PMC article. Review. - Myelin damage and repair in pathologic CNS: challenges and prospects.
Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Alizadeh A, et al. Front Mol Neurosci. 2015 Jul 27;8:35. doi: 10.3389/fnmol.2015.00035. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26283909 Free PMC article. Review. - Iron efflux from astrocytes plays a role in remyelination.
Schulz K, Kroner A, David S. Schulz K, et al. J Neurosci. 2012 Apr 4;32(14):4841-7. doi: 10.1523/JNEUROSCI.5328-11.2012. J Neurosci. 2012. PMID: 22492039 Free PMC article.
References
- Akiyama, K., S. Ichinose, A. Omori, Y. Sakurai, and H. Asou. 2002. Study of expression of myelin basic proteins (MBPs) in developing rat brain using a novel antibody reacting with four major isoforms of MBP. J. Neurosci. Res. 68:19–28. - PubMed
- Baker, J., J.P. Liu, E.J. Robertson, and A. Efstratiadis. 1993. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 75:73–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical