Extracellular signal-regulated kinase 1/2 is required for the induction of group I metabotropic glutamate receptor-mediated epileptiform discharges - PubMed (original) (raw)
Extracellular signal-regulated kinase 1/2 is required for the induction of group I metabotropic glutamate receptor-mediated epileptiform discharges
Wangfa Zhao et al. J Neurosci. 2004.
Abstract
Transient stimulation of group I metabotropic glutamate receptors (mGluRs) induces persistent prolonged epileptiform discharges in hippocampal slices via a protein synthesis-dependent process. At present, the signaling process underlying the induction of these epileptiform discharges remains unknown. We examined the possible role of extracellular signal-regulated kinases (ERK1 and ERK2) because these kinases can be activated by group I mGluRs, and their activation may regulate gene expression and alter protein synthesis. The group I mGluR agonist (S)-3,5-dihydroxyphenylglycine (DHPG; 50 microm) induced activation of ERK1/2 in hippocampal slices. 2-(2-Diamino-3-methoxyphenyl-4H-1-benzopyran-4-one (PD98059) (50 microm) a specific inhibitor of mitogen-activated protein kinase kinase (MEK), suppressed ERK1/2 activation by DHPG. PD98059 or another MEK inhibitor, 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene (10 microm), also prevented the induction of the prolonged epileptiform discharges by DHPG. In the presence of ionotropic glutamate receptor inhibitors and tetrodotoxin (blockers), DHPG-induced epileptiform discharges were suppressed, whereas ERK1/2 activation persisted. Protein kinase C inhibitors (2-[1-(3-dimethylaminopropyl)-5-methoxyindol-3-yl]-3-(1H-indol-3-yl) maleimide, 1 microm; or chelerythrine, 10 microm) did not prevent the generation of DHPG-induced epileptiform discharges, nor did they suppress the activation of ERK1/2 by DHPG in slices pretreated with the blockers. Genistein (30 microm), a broad-spectrum tyrosine kinase inhibitor, suppressed the DHPG-induced epileptiform discharges and the ERK1/2 activation in the presence of blockers. Induction of DHPG-mediated epileptiform discharges was also suppressed by 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-D]pyrimidine (10 microm), an Src-family tyrosine kinase inhibitor. The study shows that group I mGluRs activate ERK1/2 through a tyrosine kinase-dependent process and that this activation of ERK1/2 is necessary for the induction of prolonged epileptiform discharges in the hippocampus.
Figures
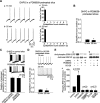
Figure 1.
DHPG-induced prolonged epileptiform discharge in hippocampal slices is associated with an increase in the phosphorylation of ERK1/2. Aa-d, Intracellular recordings from the same CA3 pyramidal cell. Ab, DHPG (50 μ
m
) caused neuronal depolarization and the appearance of clusters of synaptic depolarizations. Ac, Rhythmic short epileptiform bursts (120-800 msec) were observed 15 min after the application of DHPG. Ad, Within 10 min after the appearance of short epileptiform bursts, prolonged epileptiform discharges appeared abruptly. Ae, f, Frequency histograms of epileptiform burst duration measured from records of the same cell shown in a-d during the 15-20 min (e) and 25-30 min (f) periods of DHPG perfusion. Epileptiform bursts elicited by DHPG fell into two distinct, nonoverlapping groups.Ba, Time course of the epileptiform burst duration in eight different slices recorded 15-40 min after switching perfusion to a DHPG-containing solution. Different symbols represent different experiments. Open circles are measures from the experiment shown in A. Bb, Mean ± SEM of epileptiform burst durations averaged over 5 min periods starting at 15 min (left bar) and at 25 min (right bar) of DHPG perfusion in 20 different experiments. Mean burst duration was 0.335 ± 0.021 sec at 15 min and significantly increased to 2.180 ± 0.194 sec at 25 min (*p< 0.001) because of the appearance of prolonged epileptiform discharges. Ca, Representative Western blots of samples obtained from slices expressing prolonged epileptiform discharges (including the slice whose recordings are shown in A) after 1 hr of DHPG perfusion. Top, Anti-phospho-ERK1/2 blot; bottom, anti-total ERK1/2 blot. In each panel, the top band is p44 MAPK (ERK1) and the bottom band is p42 MAPK (ERK2). Western blots are presented in the same manner in the following figures. Bb, Summary data of normalized phospho-ERK1/2 immunoreactivity. The ratio of phospho-ERK1/2 to total-ERK1/2 immunoreactivity was normalized to the control value. After the elicitation of prolonged epileptiform discharges, ERK1/2 phosphorylation increased significantly (DHPG, 300 ± 54%; n = 6; *p < 0.01 compared with the control).
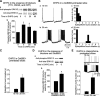
Figure 2.
MEK inhibitors block the DHPG-induced ERK1/2 phosphorylation and the induction of prolonged epileptiform discharges. A, In a slice pretreated with PD98059 (50 μ
m
; 45 min), DHPG induced short epileptiform bursts but not prolonged epileptiform discharges. In five experiments, the frequency of the short epileptiform bursts increased from 0.15 ± 0.03 Hz at 15 min of DHPG to 0.39 ± 0.10 Hz at 25 min of DHPG (p < 0.05). _B_, The mean burst duration at 25 min of DHPG perfusion (0.214 ± 0.010 sec) was significantly shorter than that at 15 min (0.273 ± 0.014 sec; _n_ = 5; *_p_ < 0.001). Compared with the control condition, PD98059 did not affect burst duration at 15 min of DHPG (_p_ = 0.16). _C_, Records from a representative CA3 pyramidal cell after 25 min of DHPG perfusion (top) and 30 min after addition of PD98059 to the perfusate (bottom). The mean duration ± SEM of prolonged epileptiform discharges in four experiments (histogram) shows that PD98059 applied after the appearance of prolonged epileptiform discharges (i.e., epileptiform bursts >1.5 sec) did not affect the occurrence of the events (25 min DHPG, 5.1 ± 0.3 sec; 30 min after PD98059 addition, 5.0 ± 0.6 sec; p = 0.77). D, Records from one CA3 neuron (top) and average duration from three experiments (histogram) of epileptiform bursts induced by DHPG in slices pretreated with another MEK inhibitor, U0126 (10 μ
m
; 45 min). Like PD98059, U0126 prevented the induction of prolonged epileptiform discharges, decreased the duration of the short epileptiform bursts (15 min, 0.274 ± 0.045 sec; 25 min, 0.179 ± 0.039 sec; *p < 0.05), and increased the frequency of the short epileptiform bursts (at 15 min, 0.12 ± 0.06 Hz; at 25 min, 0.38 ± 0.10 Hz; p < 0.05). Ea, b, Representative Western blots of slices recorded under the indicated conditions (including the slices whose recordings are shown in A and D). Ec, Summary data of normalized phospho-ERK1/2 immunoreactivity under the conditions indicated in Ea, b. Measures of the ERK1/2 phosphorylation rise by DHPG in the control condition are from Figure 1. PD98059 and U0126 significantly decreased ERK1/2 basal level (p < 0.01 and p < 0.05 compared with control, respectively) and blocked the DHPG-induced ERK1/2 phosphorylation (PD98059, 36 ± 12% of control; PD98059+DHPG, 39 ± 4%; n = 6; p = 0.8; U0126, 33 ± 11%; U0126+DHPG, 30 ± 14%; n = 3; p = 0.72).
Figure 3.
DHPG-induced prolonged epileptiform discharges, as well as ERK1/2 phosphorylation in TTX, CNQX, and CPP, persist in the presence of protein kinase C inhibitors. Aa, Representative Western blots showing the time course of ERK1/2 phosphorylation in response to DHPG applied in the presence of the blockers TTX (0.3 μ
m
), CNQX (20 μ
m
), and CPP (20 μ
m
). Ab, Summary data showing that ERK1/2 phosphorylation peaked within 20 min after DHPG application (5 min, 167 ± 12%; 20 min, 147 ± 21%; 90 min, 112 ± 7%; n = 8; *p < 0.01 compared with 0 min). B, In slices pretreated with Gö6983 (1 μ
m
; a PKC inhibitor), DHPG induced prolonged epileptiform discharges as in control. C, The average burst duration at 25 min of DHPG of perfusion (1.649 ± 0.139 sec) was significantly higher than that at 15 min of DHPG (0.382 ± 0.064 sec; *p < 0.01; n = 4). Compared with control, the presence of Gö6983 did not significantly affect burst duration (15 min, p = 0.38; 25 min, p = 0.25). D, Western blot (a) and summary data (b) of ERK1/2 activation by DHPG applied in the presence of blockers (TTX, CNQX, and CPP) in slices pretreated with Gö6983. At 5 min of DHPG application in the presence of blockers, ERK1/2 phosphorylation increased significantly over the baseline (*p < 0.01) when Gö6983 was present in the bath. This increase was comparable with that induced by DHPG at 5 min in the blockers and in the absence of Gö6983 (DHPG alone, 167 ± 12%; n = 8; Gö6983+DHPG, 166 ± 9%; n = 5; p = 0.94). E, Pretreatment of the slices with another PKC inhibitor, chelerythrine (10 μ
m
; 45 min) did not prevent the induction of short epileptiform bursts (15 min, 0.290 ± 0.023 sec) and the appearance of prolonged epileptiform discharges (25 min, 1.852 ± 0.274 sec; n = 5; *p < 0.01).
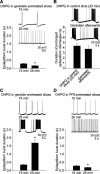
Figure 5.
Time course of mGluR-dependent phosphorylation of ERK1/2 in the presence and absence of TTX and iGlu inhibitors. A, Time course of ERK1/2 phosphorylation in response to DHPG applied in normal solution (a, representative Western blots; c, filled circles, summary data) or in the presence of TTX, CNQX, and CPP (b, blockers, representative Western blots; c, open circles, summary data). In ACSF, DHPG induced a significant increase of ERK1/2 phosphorylation that peaked at 5 min (241 ± 42%; n = 4; p < 0.01). In the presence of the blockers, DHPG still induced a peak increase of ERK1/2 phosphorylation at 5 min (167 ± 12%; n = 8; p < 0.01), although to a significantly less extent than in ACSF (p < 0.05). B, Effects of genistein pretreatment (30 μ
m
; 45 min) on DHPG-induced ERK1/2 phosphorylation in ACSF (a, Western blots; c, filled circles, summary data) and in the presence of the blockers (b, Western blots; c, open circles, summary data). The peak increase in ERK1/2 phosphorylation by DHPG at 5 min was still observed in ACSF (200 ± 33%; n = 4; p < 0.05), but it was completely prevented in the presence of the blockers (92 ± 21%; n = 5; p = 0.31). C, In chelerythrine-pretreated slices (10 μ
m
; 45 min), DHPG induced similar increases of ERK1/2 phosphorylation at 5 min in ACSF (a, Western blots; c, filled circles, summary data; at 5 min: 146 ± 13%; n = 4; p < 0.01) and in the presence of TTX, CNQX, and CPP (b, Western blots; c, open circles, summary data; at 5 min, 152 ± 6%; n = 4; p < 0.01). In ACSF, the increase at 5 min (146 ± 13%) was reduced compared with that observed in control slices (241 ± 42%; p < 0.05), whereas in the blockers the increase at 5 min (152 ± 6%) was similar to that observed in control slices (167 ± 12%; p = 0.43).
Figure 4.
Inhibitors of tyrosine kinase prevent the generation of prolonged epileptiform discharges. A, Genistein (30 μ
m
), a broad-spectrum tyrosine kinase inhibitor, introduced 45 min before adding DHPG, prevented the appearance of prolonged epileptiform discharges. DHPG still accelerated the frequency of short epileptiform bursts (top, on average from 0.11 ± 0.02 Hz at 15 min of DHPG to 0.36 ± 0.06 Hz at 25 min of DHPG; n = 8; p < 0.01). The burst duration at 25 min of DHPG (0.163 ± 0.023 sec) was significantly shorter than that at 15 min DHPG (0.288 ± 0.030 sec; n = 8; *p < 0.01). B, Genistein applied after the appearance of DHPG-mediated prolonged epileptiform discharges did not affect the occurrence of the events (25 min DHPG, 4.4 ± 0.4 sec; 30 min after genistein addition, 3.8 ± 0.6 sec; n = 6; p = 0.25). C, In contrast to genistein, pretreatment of slices with its inactive analog, genistin (30 μ
m
; 45 min), did not prevent the induction of prolonged epileptiform discharges by DHPG (epileptiform burst duration at 15 min, 0.352 ± 0.046 sec; at 25 min, 1.687 ± 0.197 sec; n = 4; *p < 0.01). D, Similar to genistein, pretreatment with the selective Src-family tyrosine kinase inhibitor PP2 (10 μ
m
; 45 min) prevented the induction of prolonged epileptiform discharges by DHPG. Short epileptiform bursts were still induced (top) and their duration decreased from 0.282 ± 0.043 sec (15 min) to 0.184 ± 0.024 sec (25 min; n = 3; *p < 0.05). The frequency of the short epileptiform bursts increased from 0.09 ± 0.03 Hz at 15 min of DHPG to 0.44 ± 0.10 Hz at 25 min of DHPG (n = 3; p < 0.05). The presence of tyrosine kinase inhibitors did not affect the duration of short epileptiform bursts at 15 min (compared with control at 15 min: genistein, p = 0.23; PP2, p = 0.36).
Similar articles
- Phosphorylation of mitogen-activated protein kinase in cultured rat cortical glia by stimulation of metabotropic glutamate receptors.
Peavy RD, Conn PJ. Peavy RD, et al. J Neurochem. 1998 Aug;71(2):603-12. doi: 10.1046/j.1471-4159.1998.71020603.x. J Neurochem. 1998. PMID: 9681450 - Group I mGluRs coupled to G proteins are regulated by tyrosine kinase in dopamine neurons of the rat midbrain.
Tozzi A, Guatteo E, Caputi L, Bernardi G, Mercuri NB. Tozzi A, et al. J Neurophysiol. 2001 Jun;85(6):2490-7. doi: 10.1152/jn.2001.85.6.2490. J Neurophysiol. 2001. PMID: 11387395 - Group I metabotropic glutamate receptor-mediated gene expression in striatal neurons.
Mao LM, Zhang GC, Liu XY, Fibuch EE, Wang JQ. Mao LM, et al. Neurochem Res. 2008 Oct;33(10):1920-4. doi: 10.1007/s11064-008-9654-4. Epub 2008 Mar 20. Neurochem Res. 2008. PMID: 18351459 Review. - Glutamate Receptors in Epilepsy: Group I mGluR-Mediated Epileptogenesis.
Bianchi R, Wong RKS, Merlin LR. Bianchi R, et al. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies [Internet]. 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012. PMID: 22787676 Free Books & Documents. Review.
Cited by
- Dynamic seizure-related changes in extracellular signal-regulated kinase activation in a mouse model of temporal lobe epilepsy.
Houser CR, Huang CS, Peng Z. Houser CR, et al. Neuroscience. 2008 Sep 22;156(1):222-37. doi: 10.1016/j.neuroscience.2008.07.010. Epub 2008 Jul 10. Neuroscience. 2008. PMID: 18675888 Free PMC article. - Unique signaling profiles of positive allosteric modulators of metabotropic glutamate receptor subtype 5 determine differences in in vivo activity.
Rook JM, Noetzel MJ, Pouliot WA, Bridges TM, Vinson PN, Cho HP, Zhou Y, Gogliotti RD, Manka JT, Gregory KJ, Stauffer SR, Dudek FE, Xiang Z, Niswender CM, Daniels JS, Jones CK, Lindsley CW, Conn PJ. Rook JM, et al. Biol Psychiatry. 2013 Mar 15;73(6):501-9. doi: 10.1016/j.biopsych.2012.09.012. Epub 2012 Nov 7. Biol Psychiatry. 2013. PMID: 23140665 Free PMC article. - APP Causes Hyperexcitability in Fragile X Mice.
Westmark CJ, Chuang SC, Hays SA, Filon MJ, Ray BC, Westmark PR, Gibson JR, Huber KM, Wong RK. Westmark CJ, et al. Front Mol Neurosci. 2016 Dec 15;9:147. doi: 10.3389/fnmol.2016.00147. eCollection 2016. Front Mol Neurosci. 2016. PMID: 28018172 Free PMC article. - Biotransformation of a novel positive allosteric modulator of metabotropic glutamate receptor subtype 5 contributes to seizure-like adverse events in rats involving a receptor agonism-dependent mechanism.
Bridges TM, Rook JM, Noetzel MJ, Morrison RD, Zhou Y, Gogliotti RD, Vinson PN, Xiang Z, Jones CK, Niswender CM, Lindsley CW, Stauffer SR, Conn PJ, Daniels JS. Bridges TM, et al. Drug Metab Dispos. 2013 Sep;41(9):1703-14. doi: 10.1124/dmd.113.052084. Epub 2013 Jul 2. Drug Metab Dispos. 2013. PMID: 23821185 Free PMC article. - Extracellular glutamate exposure facilitates group I mGluR-mediated epileptogenesis in the hippocampus.
Zhao W, Chuang SC, Young SR, Bianchi R, Wong RK. Zhao W, et al. J Neurosci. 2015 Jan 7;35(1):308-15. doi: 10.1523/JNEUROSCI.1944-14.2015. J Neurosci. 2015. PMID: 25568123 Free PMC article.
References
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S (1992) Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem 267: 13361-13368. - PubMed
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y (1987) Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262: 5592-5595. - PubMed
- Anwyl R (1999) Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev 29: 83-120. - PubMed
- Bading H, Ginty DD, Greenberg ME (1993) Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 260: 181-186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous