Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis - PubMed (original) (raw)
Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis
Samuel T Waters et al. J Exp Med. 2004.
Abstract
In lupus-prone NZM2328 mice, a locus Cgnz1 on chromosome 1 was linked to chronic glomerulonephritis, severe proteinuria, and early mortality in females. A locus Adnz1 on chromosome 4 was linked to antinuclear antibody (ANA) and anti-double stranded DNA (dsDNA) antibody (Ab) production. In this investigation, two congenic strains, NZM2328.C57L/Jc1 (NZM.C57Lc1) and NZM2328.C57L/Jc4 (NZM.C57Lc4), were generated by replacing the respective genetic intervals containing either Cgnz1 or Adnz1 with those from C57L/J, a nonlupus-prone strain. The NZM.C57Lc1 females had markedly reduced incidence of chronic glomerulonephritis and severe proteinuria. NZM.C57Lc4 females had chronic glomerulonephritis and severe proteinuria without circulating ANA, anti-dsDNA, and antinucleosome Ab. These data confirm the linkage analysis. Unexpectedly, NZM.C57Lc1 females had little anti-dsDNA and related Ab, suggesting the presence of a second locus Adnz2 on chromosome 1. The diseased NZM.C57Lc4 kidneys had immune complexes by immunofluorescence and electron microscopy. The eluates from these kidneys did not contain ANA, anti-dsDNA, and antinucleosome Ab, indicative of the presence of non-anti-dsDNA nephritogenic Ab. Thus, breaking tolerance to dsDNA and chromatin is not required for the pathogenesis of lupus nephritis. These results reaffirm that anti-dsDNA and related Ab production and chronic glomerulonephritis are under independent genetic control. These findings have significant implications in the pathogenesis of systemic lupus erythematosus.
Figures
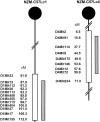
Figure 1.
NZM.C57Lc1 and NZM.C57Lc4 congenic lines were derived by replacing the genetic intervals in NZM2328 with those from C57L/J (hatched bars). The genetic intervals with SLE susceptibility genes in NZM2328 delineated by informative microsatellite markers are shown (open bars). Chromosome intervals are not drawn to scale.
Figure 2.
Development of severe proteinuria in females of NZM2328 and NZM.C57Lc4 but not in those of NZM.C57Lc1. (A) Incidence of severe proteinuria in each strain of mice. (B) Kinetics of proteinuria development.
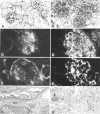
Figure 3.
Histological, immunofluorescence, and EM studies of representative kidneys from NZM.C57Lc1 and NZM.C57Lc4 female mice. (A) Normal glomeruli (hematoxylin and eosin staining, ×200) are seen in NZM.C57Lc1. (B) In contrast, in the NZM.C57Lc4 congenic, enlarged glomeruli with mesangial proliferation, hypercellularity, obliterated capillary loops, and glomerulosclerosis are evident. (C) Immunofluorescence studies show some mesangial IgG deposits in NZM.C57Lc1, similar to the pattern seen in aged C57L/J. (D) A coarsely granular staining pattern of IgG deposits in both the mesangia and peripheral capillary walls of the glomeruli of NZM.C57Lc4. (E) Staining of the Bowman capsule and mesangia with anti-C3 Ab are seen in NZM.C57Lc1. (F) Coarsely granular staining by anti-C3 Ab throughout the glomeruli is seen in NZM.C57Lc4. (G) EM study shows normal glomeruli without electron-dense deposits in the subepithelial or subendothelial spaces (×10,000) in the kidney of NZM.C57Lc1. (H) In comparison, electron-dense deposits in both subendothelial space (arrow) and the mesangia (arrowheads) in the glomeruli of NZM.C57Lc4 are readily detected.
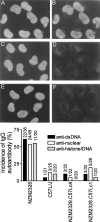
Figure 4.
Marked reduction of circulating anti-dsDNA, antinuclear, and antinucleosome Ab in NZM.C57Lc1 and NZM.C57Lc4 congenic lines in comparison with NZM2328. Staining of HeLa cell nuclei by DAPI are seen in A, C, and E. The right side of the figure shows the presence of ANA in the serum of NZM2328 (B) but not in the sera of NZM.C57Lc1 (D) and NZM.C57Lc4 (F). Although not shown, the majority of the sera from C57L/J were not positive for ANA. On the bottom, frequencies of the presence of anti-dsDNA, antinuclear, and antihistone/DNA Ab in these strains are summarized.
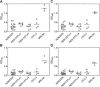
Figure 5.
Lack of RF in sera of NZM2328 and its congenics. Human IgG was used as the substrate in A and C to detect anti-IgG activities. Mouse sera were used at a dilution of 1:50 for A and 1:250 for C. Rabbit IgG was used as the substrate in B and D with mouse sera diluted at 1:50 and 1:250, respectively. A pool of MRL/_lpr+/+_sera was used as a positive control showing readily detectable RF in this strain of mice.
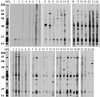
Figure 6.
Western blot analysis to detect autoantibodies to cellular constituents in sera of NZM2328 and its congenics. Cell lysate of WEHI 7.1 lymphoma cell line was used as the substrate with sera at the dilution of 1:50. On the top, sera from mice at 4–5 mo of age were used with MRL/lpr+/+(MRL) pooled sera as the positive control. Lanes 1–8, NZM.C57Lc1; lanes 9–15, NZM.C57Lc4; lanes 16–22, NZM2328. On the bottom, sera at death or at death at the age of 12 mo were used. Lanes 1–6, NZM.C57Lc1; lanes 7–13, NZM.C57Lc4; lanes 14–20, NZM2328; lanes 21–24, C57L/J.
Figure 7.
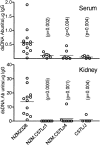
Anti-dsDNA Ab in sera and kidney eluates from NZM2328, NZM.C57Lc1, NZM.C57Lc4, and C57L/J females at 11–12 mo. Abs to dsDNA were present in sera of NZM2328 and they were enriched in their kidney eluates. These Abs were rarely detected in the sera and the kidney eluates of the other three strains. P-values indicate significant differences of the respective strain as compared with NZM2328.
Figure 8.
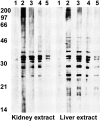
Abs eluted from nephritic kidneys of NZM2328 and NZM.C57Lc4 mice are reactive with proteins within the kidney and liver extracts. Proteins in the kidney (left) and liver (right) extracts were separated on a 12% SDS-PAGE, transferred to nitrocellulose paper, and used for analyzing the reactivity of Abs eluted from the nephritic kidneys of NZM2328 (lanes 2 and 3) and NZM.C57Lc4 (lanes 4 and 5) mice. Each lane is equivalent to 60 μg total protein. Abs were used at a concentration of 30 (lanes 2 and 4) and 10 μg/ml (lanes 3 and 5). Lane 1 in both panels represents reactivity of the goat anti–mouse IgG–horseradish peroxidase conjugate with the extracts. Numbers on the left represent molecular weights in kilodaltons.
Figure 9.
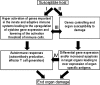
Interactive model for the pathogenesis of SLE. Pathway I, autoantibody production and activation of effector T cells and pathway II, activation of susceptibility genes and end organ damage, can be initiated independently while they interact at different levels as indicated by pathways III and IV. The interactions between these pathways lead to end organ damage.
Similar articles
- NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci.
Waters ST, Fu SM, Gaskin F, Deshmukh US, Sung SS, Kannapell CC, Tung KS, McEwen SB, McDuffie M. Waters ST, et al. Clin Immunol. 2001 Sep;100(3):372-83. doi: 10.1006/clim.2001.5079. Clin Immunol. 2001. PMID: 11513551 - Nucleosome-restricted antibodies are detected before anti-dsDNA and/or antihistone antibodies in serum of MRL-Mp lpr/lpr and +/+ mice, and are present in kidney eluates of lupus mice with proteinuria.
Amoura Z, Chabre H, Koutouzov S, Lotton C, Cabrespines A, Bach JF, Jacob L. Amoura Z, et al. Arthritis Rheum. 1994 Nov;37(11):1684-8. doi: 10.1002/art.1780371118. Arthritis Rheum. 1994. PMID: 7980678 - Genetic approach to study lupus glomerulonephritis.
Ge Y, Brown MG, Wang H, Fu SM. Ge Y, et al. Methods Mol Biol. 2012;900:271-90. doi: 10.1007/978-1-60761-720-4_13. Methods Mol Biol. 2012. PMID: 22933074 Free PMC article. - [Role of the nucleosome in the physiopathology of systemic lupus erythematosus].
Amoura Z, Piette JC. Amoura Z, et al. Ann Med Interne (Paris). 2003 Feb;154(1):25-32. Ann Med Interne (Paris). 2003. PMID: 12746656 Review. French. - Autoimmunity against nucleosomes and lupus nephritis.
Van Bruggen MC, Kramers C, Berden JH. Van Bruggen MC, et al. Ann Med Interne (Paris). 1996;147(7):485-9. Ann Med Interne (Paris). 1996. PMID: 9092359 Review.
Cited by
- Lupus nephritis: enigmas, conflicting models and an emerging concept.
Seredkina N, Van Der Vlag J, Berden J, Mortensen E, Rekvig OP. Seredkina N, et al. Mol Med. 2013 Jul 24;19(1):161-9. doi: 10.2119/molmed.2013.00010. Mol Med. 2013. PMID: 23752208 Free PMC article. Review. - The role of anti-alpha-actinin antibodies in the pathogenesis and monitoring of lupus nephritis.
Youinou P, Putterman C. Youinou P, et al. Arthritis Res Ther. 2009;11(6):137. doi: 10.1186/ar2869. Epub 2009 Dec 11. Arthritis Res Ther. 2009. PMID: 20017900 Free PMC article. - A Central Role for HLA-DR3 in Anti-Smith Antibody Responses and Glomerulonephritis in a Transgenic Mouse Model of Spontaneous Lupus.
Chowdhary VR, Dai C, Tilahun AY, Hanson JA, Smart MK, Grande JP, Rajagopalan G, Fu SM, David CS. Chowdhary VR, et al. J Immunol. 2015 Nov 15;195(10):4660-7. doi: 10.4049/jimmunol.1501073. Epub 2015 Oct 16. J Immunol. 2015. PMID: 26475924 Free PMC article. - The role of CD4CD25 T cells in autoantibody production in murine lupus.
Hsu WT, Suen JL, Chiang BL. Hsu WT, et al. Clin Exp Immunol. 2006 Sep;145(3):513-9. doi: 10.1111/j.1365-2249.2006.03173.x. Clin Exp Immunol. 2006. PMID: 16907921 Free PMC article. - Anti-alpha8 integrin immunoliposomes in glomeruli of lupus-susceptible mice: a novel system for delivery of therapeutic agents to the renal glomerulus in systemic lupus erythematosus.
Scindia Y, Deshmukh U, Thimmalapura PR, Bagavant H. Scindia Y, et al. Arthritis Rheum. 2008 Dec;58(12):3884-91. doi: 10.1002/art.24026. Arthritis Rheum. 2008. PMID: 19035491 Free PMC article.
References
- Tan, E.M., A.S. Cohen, J.F. Fries, A.T. Masi, D.J. McShane, N.F. Rothfield, J.G. Schaller, N. Talal, and R.J. Winchester. 1982. The 1982 revised criteria for the classification of systemic lupus erythematosus (SLE). Arthritis Rheum. 25:1271–1277. - PubMed
- Hochberg, M.C. 1977. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus (letter). Arthritis Rheum. 40:1725. - PubMed
- Emlen, W., D.S. Pisetsky, and R.P. Taylor. 1986. Antibodies to DNA. Arthritis Rheum. 29:1417–1425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AR045222/AR/NIAMS NIH HHS/United States
- P50 AR-45222/AR/NIAMS NIH HHS/United States
- R01 AR-42027/AR/NIAMS NIH HHS/United States
- R01 AR047988/AR/NIAMS NIH HHS/United States
- R01 AR-42465/AR/NIAMS NIH HHS/United States
- R01 AR-47988/AR/NIAMS NIH HHS/United States
- P50AR-45222/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases