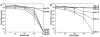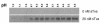The mature avian leukosis virus subgroup A envelope glycoprotein is metastable, and refolding induced by the synergistic effects of receptor binding and low pH is coupled to infection - PubMed (original) (raw)
The mature avian leukosis virus subgroup A envelope glycoprotein is metastable, and refolding induced by the synergistic effects of receptor binding and low pH is coupled to infection
Jason G Smith et al. J Virol. 2004 Feb.
Abstract
The spring-loaded model stipulates that influenza virus infection is coupled to the transition of the virus hemagglutinin (HA) from a metastable conformation to a highly stable conformation at low pH. The properties of retrovirus envelope glycoproteins indicate that infection is coupled to an analogous conformational change. As a test of this hypothesis, the requirements for avian leukosis virus A (ALV-A) infection were examined. These studies indicate that, like HA, the conformation of the mature ALV-A envelope glycoprotein is metastable and that infection is linked to refolding at low pH. However, unlike HA, low-pH activation is only observed after priming by receptor. Therefore, ALV-A infection is dependent on the synergistic effects of receptor binding and low pH, suggesting that receptor binding superimposes an additional constraint on activation of ALV-A fusion that proceeds by a mechanism comparable to that of influenza virus.
Figures
FIG. 1.
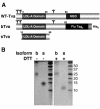
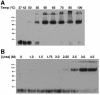
Tva isoforms. (A) Schematic diagram of Tva-derived proteins used in this study. Numbers refer to the positions of residues in mature wild-type (WT) Tva. MSD, membrane-spanning domain. Flu Tag3, three copies of an influenza virus HA epitope tag. Branched symbols denote positions of N-linked glycosylation sites. (B) Coomassie R-250-stained gels of purified bTva and sTva. Where noted, samples were boiled in 100 mM DTT prior to loading.
FIG. 2.
sTva and bTva are functional virus receptors. RCASBP(A)-EGFP virus was incubated with bTva (A) or sTva (B) at the indicated concentrations and spinoculated onto 293 (⋄) or 293-Tva (□) cells as described in Materials and Methods. Forty-eight hours later, cells were analyzed for acquired EGFP expression by flow cytometry, and virus titers (infectious units per milliliter) were determined by end point dilution. Error bars represent the standard deviation of three measurements.
FIG. 3.
In vitro inactivation of ALV-A infection by sTva and bTva is dependent upon exposure to acid pH. RCASBP(A)-EGFP virus was exposed to bTva (A) or sTva (B) at the indicated concentrations. After a 20-min incubation on ice, the pH was adjusted to 5.0 (⋄), 5.2 (□), 5.5 (▵), 6.0 (×), 6.8 (*), or 7.5 (○), and samples were incubated for an additional 30 min at 37°C. After neutralization, virus-receptor complexes were spinoculated onto 293-Tva cells, and these cells were analyzed for acquired EGFP expression 48 h later. Viral titers (infectious units per milliliter) were determined by end point dilution. Error bars represent the standard deviation of three measurements.
FIG. 4.
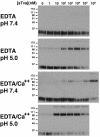
Acid pH and calcium increase the specific activity of sTva for inducing a conformational change in ALV-A TM. RCASBP(A)-EGFP virus was incubated with increasing concentrations of sTva. After incubation, samples were exposed to DTT and loaded onto SDS-PAGE gels without boiling. All experiments were performed in 100 μM EDTA, and 1.1 mM CaCl2 was added where indicated. Formation of the 90- and >170-kDa species indicative of the postfusion conformation of TM was determined by immunoblotting (TMCA). Note that the 90-kDa and >170-kDa species observed in this experiment are equivalent to the 70- and 150-kDa species described in our previous report (25). The discrepancy in assigned molecular masses is dependent upon the concentration of polyacrylamide in SDS-PAGE. In lower-percentage gels (4 to 15% gradient gels and 10% gels), the oligomeric TM species migrate at approximately 70 and 150 kDa (; data not shown). However, in the 13% gels shown here, they migrate at approximately 90 and >170 kDa.
FIG. 5.
Determination of the pH threshold for sTva-induced conformational change in ALV-A TM. RCASBP(A)-EGFP virus was incubated with or without 20 nM sTva in the presence of 100 μM EDTA and 1.1 mM CaCl2 at the indicated pHs and analyzed by TMCA as described in the legend to Fig. 4. Only the 90-kDa species of TM is shown.
FIG. 6.
The presence of lipid targets does not alter the receptor and pH requirements for conformational change in ALV-A TM. RCASBP(A)-EGFP virus was incubated with increasing concentrations of sTva in the presence or absence of liposomes at pH 5.0 or 7.4 and analyzed for the presence of the 90- and >170-kDa isoforms by TMCA as described in the legend to Fig. 4.
FIG. 7.
Mild denaturation induces the formation of oligomeric ALV-A TM. RCASBP(A)-EGFP virus was incubated at the indicated temperatures for 30 min or boiled for 5 min (A) or incubated at 37°C in the presence of increasing concentrations of urea (B) and analyzed by TMCA as described in the legend to Fig. 4.
FIG. 8.
Determination of the effects of sTva and acid pH on the thermal threshold for triggering conformational change in ALV-A TM. RCASBP(A)-EGFP virus was incubated at the indicated temperatures in the presence or absence of 100 nM sTva at pH 5.0 or 7.4 and analyzed by TMCA as described in the legend to Fig. 4. Only the 90-kDa species of TM is shown.
Similar articles
- Receptor-induced conformational changes in the SU subunit of the avian sarcoma/leukosis virus A envelope protein: implications for fusion activation.
Delos SE, Godby JA, White JM. Delos SE, et al. J Virol. 2005 Mar;79(6):3488-99. doi: 10.1128/JVI.79.6.3488-3499.2005. J Virol. 2005. PMID: 15731243 Free PMC article. - Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein.
Mothes W, Boerger AL, Narayan S, Cunningham JM, Young JA. Mothes W, et al. Cell. 2000 Nov 10;103(4):679-89. doi: 10.1016/s0092-8674(00)00170-7. Cell. 2000. PMID: 11106737 - Alpharetrovirus envelope-receptor interactions.
Barnard RJ, Young JA. Barnard RJ, et al. Curr Top Microbiol Immunol. 2003;281:107-36. doi: 10.1007/978-3-642-19012-4_3. Curr Top Microbiol Immunol. 2003. PMID: 12932076 Review. - Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin.
Skehel JJ, Wiley DC. Skehel JJ, et al. Annu Rev Biochem. 2000;69:531-69. doi: 10.1146/annurev.biochem.69.1.531. Annu Rev Biochem. 2000. PMID: 10966468 Review.
Cited by
- Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme.
White JM, Delos SE, Brecher M, Schornberg K. White JM, et al. Crit Rev Biochem Mol Biol. 2008 May-Jun;43(3):189-219. doi: 10.1080/10409230802058320. Crit Rev Biochem Mol Biol. 2008. PMID: 18568847 Free PMC article. Review. - Small interfering RNA profiling reveals key role of clathrin-mediated endocytosis and early endosome formation for infection by respiratory syncytial virus.
Kolokoltsov AA, Deniger D, Fleming EH, Roberts NJ Jr, Karpilow JM, Davey RA. Kolokoltsov AA, et al. J Virol. 2007 Jul;81(14):7786-800. doi: 10.1128/JVI.02780-06. Epub 2007 May 9. J Virol. 2007. PMID: 17494077 Free PMC article. - Stabilization of TM trimer interactions during activation of moloney murine leukemia virus Env.
Sjöberg M, Lindqvist B, Garoff H. Sjöberg M, et al. J Virol. 2008 Mar;82(5):2358-66. doi: 10.1128/JVI.01931-07. Epub 2007 Dec 19. J Virol. 2008. PMID: 18094169 Free PMC article. - The fusion-controlling disulfide bond isomerase in retrovirus Env is triggered by protein destabilization.
Wallin M, Ekström M, Garoff H. Wallin M, et al. J Virol. 2005 Feb;79(3):1678-85. doi: 10.1128/JVI.79.3.1678-1685.2005. J Virol. 2005. PMID: 15650193 Free PMC article. - Stable association of herpes simplex virus with target membranes is triggered by low pH in the presence of the gD receptor, HVEM.
Whitbeck JC, Zuo Y, Milne RS, Cohen GH, Eisenberg RJ. Whitbeck JC, et al. J Virol. 2006 Apr;80(8):3773-80. doi: 10.1128/JVI.80.8.3773-3780.2006. J Virol. 2006. PMID: 16571794 Free PMC article.
References
- Bates, P., J. A. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. - PubMed
- Blacklow, S. C., and P. S. Kim. 1996. Protein folding and calcium binding defects arising from familial hypercholesterolemia mutations of the LDL receptor. Nat. Struct. Biol. 3:758-762. - PubMed
- Boerkoel, C. F., M. J. Federspiel, D. W. Salter, W. Payne, L. B. Crittenden, H. J. Kung, and S. H. Hughes. 1993. A new defective retroviral vector system based on the Bryan strain of Rous sarcoma virus. Virology 195:669-679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007498/AI/NIAID NIH HHS/United States
- T32 HL007623/HL/NHLBI NIH HHS/United States
- T32-AI07498/AI/NIAID NIH HHS/United States
- T32-HL07623/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials