Loss of the anaphase-promoting complex in quiescent cells causes unscheduled hepatocyte proliferation - PubMed (original) (raw)
Loss of the anaphase-promoting complex in quiescent cells causes unscheduled hepatocyte proliferation
Karin G Wirth et al. Genes Dev. 2004.
Abstract
The anaphase-promoting complex or cyclosome (APC/C) is an ubiquitin protein ligase that together with Cdc20 and Cdh1 targets mitotic proteins for degradation by the proteosome. APC-Cdc20 activity during mitosis triggers anaphase by destroying securin and cyclins. APC-Cdh1 promotes degradation of cyclins and other proteins during G(1). We show that loss of APC/C during embryogenesis is early lethal before embryonic day E6.5 (E6.5). To investigate the role of APC/C in quiescent cells, we conditionally inactivated the subunit Apc2 in mice. Deletion of Apc2 in quiescent hepatocytes caused re-entry into the cell cycle and arrest in metaphase, resulting in liver failure. Re-entry into the cell cycle either occurred without any proliferative stimulus or could be easily induced. We demonstrate that the APC has an additional function to prevent hepatocytes from unscheduled re-entry into the cell cycle.
Figures
Figure 1.
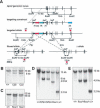
Generation of a conditional allele for Apc2. (A) Targeting strategy for generating Apc2 floxed and Δ alleles. Representation of the Apc2 genomic locus, the targeting vector, and the targeted allele. Exons are shown as black boxes. For Apc2, exons 2–4 were flanked by loxP sites (triangles). The selection cassette Neo-Tk is indicated as red and green boxes and the DTA cassette as a blue box. The selection cassettes were removed by _Flpe_-mediated recombination (dashed lines) to obtain floxed alleles, and _Cre_-mediated recombination was used to generate Δ alleles. (B) Southern blot analysis to check for integration of the targeting vector at the Apc2 genomic locus. EcoRV-restriction sites for Apc2 and the external probe a1 were used. (C) Southern blot to determine integration of the third loxP site by EcoRI-restriction-digest and hybridization with the internal probe b1. (D) Southern blot of targeted ES cells after transient transfection with an _Flpe_- or _Cre_-recombinase plasmid to delete the selection cassettes or generate Δ alleles. Diagnostic sites are EcoRV, and c1 is an internal probe. (E) Southern blot to confirm germ-line transmission of Apc2 flox and Δ alleles with EcoRV-digested DNA and the internal probe c1.
Figure 2.
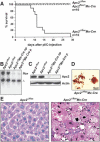
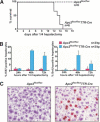
Liver failure in _Apc2_Δ/flox Mx-Cre mice. (A) Survival curve of adult _Apc2_Δ/flox Mx-Cre and control mice (_Apc2_Δ/flox and Apc2flox/+ Mx-Cre mice) after two injections of 400 μg of pI/C. Most mice died between day 11 and day 14 after the first pI/C injection. (B) Southern blot analysis of _Apc2_Δ/flox or _Apc2_Δ/flox Mx-Cre livers; (np) no phenotype, (p) with phenotype. (C) Western blot analysis of _Apc2_Δ/flox and _Apc2_Δ/flox Mx-Cre livers after pI/C injection for Apc2-protein levels. Actin is the loading control; (np) no phenotype, (p) phenotype. (D) Squashes from _Apc2_Δ/flox Mx-Cre livers. Seventy-three percent of mitotic cells were in a prometaphase-like state with condensed chromosomes. (E) Hematoxylin/eosin staining of livers from _Apc2_Δ/flox and _Apc2_Δ/flox Mx-Cre mice. _Apc2_Δ/flox Mx-Cre hepatocytes appeared larger and had condensed chromosomes.
Figure 4.
_Apc2_Δ/flox Mx-Cre hepatocytes cannot maintain liver function and arrest in mitosis. (A) PAS staining. Most _Apc2_Δ/flox Mx-Cre hepatocytes were PAS-negative as indicated by the loss of the staining. (B) Ki67 and (C) pH3 staining of _Apc2_Δ/flox and _Apc2_Δ/flox Mx-Cre livers at day 11 after pI/C injection. The majority of _Apc2_Δ/flox Mx-Cre hepatocytes were in mitosis. Arrowheads point to Ki67- or pH3-positive cells.
Figure 3.
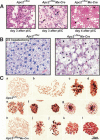
2/3 hepatectomy in transplanted _Apc2_Δ/flox Mx-Cre mice causes metaphase arrest. (A) Cytospins of bone marrow cells at day 3 and day 4 after 1 × 400 μg pI/C injection. In the _Apc2_Δ/flox Mx-Cre bone marrow, the number of mitotic cells (arrowheads) was increased at day 3 after pI/C. At day 4, only erythrocytes and a few mature lymphocytes could be detected in _Apc2_Δ/flox Mx-Cre bone marrow. (B) Hematoxylin/eosin staining of _Apc2_Δ/flox and _Apc2_Δ/flox Mx-Cre livers 72 h after 2/3 hepatectomy in unaffected _Apc2_Δ/flox Mx-Cre mice. In the _Apc2_Δ/flox Mx-Cre mice, 70% of the hepatocytes were enlarged and 50% of the cells were in metaphase. (C) Squashes from Apc2+/+ and _Apc2_Δ/flox Mx-Cre livers 48 h after 2/3 hepatectomy. (a_–_g) Mitosis in Apc2+/+ hepatocytes. (a) Prophase; (b) early prometaphase, NEB but no biorientation of chromosomes; (c) late prometaphase, not all chromosomes are aligned yet at the equatorial plate; (d) metaphase, sideview, all chromosomes were aligned at the equatorial plate; (e) anaphase, sister chromatids were segregating to opposite poles; (f) telophase; (g) early G1. (h_–_l) Mitosis in _Apc2_Δ/flox Mx-Cre hepatocytes. (h) Prophase, no difference from Apc2+/+ hepatocytes; (i) prometaphase, the shape of individual chromosomes appeared ill defined; (j) prometaphase-like cell with condensed chromatin; (k) metaphase, polar view; (l) metaphase-like cell with condensed chromatin, but individual chromosomes could not be observed.
Figure 5.
Levels of APC/C targets in _Apc2_Δ/flox Mx-Cre hepatocytes. Cryosections of _Apc2_Δ/flox and _Apc2_Δ/flox Mx-Cre livers were analyzed by indirect immunofluorescence. (A) We found that 50%–70% of _Apc2_Δ/flox Mx-Cre hepatocytes stained for Ki67, indicating that they entered from a quiescent state to a proliferative state. About 1% of Ki67-positive cells were detected in _Apc2_Δ/flox hepatocytes. (B) _Apc2_Δ/flox Mx-Cre hepatocytes with condensed chromosomes were positive for the mitotic marker pH3. A few pH3-positive cells were found in _Apc2_Δ/flox livers. (C) The mitotic regulator of the APC/C Cdc20 was expressed in _Apc2_Δ/flox Mx-Cre hepatocytes with condensed chromosomes. It localized to the spindle and the cytoplasm after nuclear envelope breakdown (arrowheads). (D) Cyclin A2 was found in mitotic _Apc2_Δ/flox Mx-Cre hepatocytes at the spindle and in the cytoplasm (arrowheads). A low percentage of _Apc2_Δ/flox Mx-Cre cells with uncondensed chromosomes as well as 1% of _Apc2_Δ/flox hepatocytes were cyclin A2 positive. (E) Western blot analysis for Apc2, cyclin A2, cyclin B1, cyclin D1, securin, p27, SnoN, Plk, and Cdc20 of liver extracts. As a positive control, mouse embryonic fibroblasts (MEF) were used. These MEFs had been synchronized by double thymidine block and were released into nocodazole. Livers from Apc2+/+, _Apc2_Δ/flox, and Apc2flox/+ Mx-Cre mice were compared with livers from _Apc2_Δ/flox Mx-Cre mice with no phenotype (np) and with phenotype (p). Tubulin is the loading control.
Figure 6.
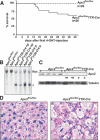
Re-entry of Apc2-deficient hepatocytes into the cell cycle is cell autonomous. (A) Survival curve of Apc2flox/flox and Apc2flox/flox TTR-Cre mice. Forty percent of the mice died of liver failure within 14 d after 4-OHT injections 1 mg/mouse i.p. five times a week and repetition of injections every other week. (B) Southern blot analysis showing deletion of the floxed allele by TTR-Cre upon injection of 4-OHT. (C) On Western blot analysis, the Apc2 protein levels were down-regulated; (np) no phenotype, (p) phenotype. (D) Histological analysis of Apc2flox/flox and affected Apc2flox/flox TTR-Cre livers by hematoxylin/eosin staining.
Figure 7.
1/4 hepatectomy induces re-entry into the cell cycle in unaffected Apc2flox/flox TTR-Cre livers. (A) Survival curve of Apc2flox/flox and Apc2flox/flox TTR-Cre mice after 1/4 hepatectomy. Apc2flox/flox TTR-Cre mice died within the second week after 1/4 hepatectomy of liver failure. (B) Quantification of Ki67- and BrdU-positive cells 24 h, 48 h, and 72 h after 1/4 hepatectomy. For each time point, three Apc2flox/flox and three Apc2flox/flox TTR-Cre mice were used. (C) Ki67 staining 48 h after 1/4 hepatectomy.
Similar articles
- Budding yeast RSI1/APC2, a novel gene necessary for initiation of anaphase, encodes an APC subunit.
Kramer KM, Fesquet D, Johnson AL, Johnston LH. Kramer KM, et al. EMBO J. 1998 Jan 15;17(2):498-506. doi: 10.1093/emboj/17.2.498. EMBO J. 1998. PMID: 9430641 Free PMC article. - The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis.
Prinz S, Hwang ES, Visintin R, Amon A. Prinz S, et al. Curr Biol. 1998 Jun 18;8(13):750-60. doi: 10.1016/s0960-9822(98)70298-2. Curr Biol. 1998. PMID: 9651679 - Methods to measure ubiquitin-dependent proteolysis mediated by the anaphase-promoting complex.
Kraft C, Gmachl M, Peters JM. Kraft C, et al. Methods. 2006 Jan;38(1):39-51. doi: 10.1016/j.ymeth.2005.07.005. Methods. 2006. PMID: 16343932 Review. - The anaphase-promoting complex/cyclosome (APC/C): cell-cycle-dependent and -independent functions.
Manchado E, Eguren M, Malumbres M. Manchado E, et al. Biochem Soc Trans. 2010 Feb;38(Pt 1):65-71. doi: 10.1042/BST0380065. Biochem Soc Trans. 2010. PMID: 20074037 Review.
Cited by
- Spatial control of the APC/C ensures the rapid degradation of cyclin B1.
Cirillo L, Young R, Veerapathiran S, Roberti A, Martin M, Abubacar A, Perosa C, Coates C, Muhammad R, Roumeliotis TI, Choudhary JS, Alfieri C, Pines J. Cirillo L, et al. EMBO J. 2024 Oct;43(19):4324-4355. doi: 10.1038/s44318-024-00194-2. Epub 2024 Aug 14. EMBO J. 2024. PMID: 39143240 Free PMC article. - APC/C prevents a noncanonical order of cyclin/CDK activity to maintain CDK4/6 inhibitor-induced arrest.
Mouery BL, Baker EM, Mei L, Wolff SC, Mills CA, Fleifel D, Mulugeta N, Herring LE, Cook JG. Mouery BL, et al. Proc Natl Acad Sci U S A. 2024 Jul 23;121(30):e2319574121. doi: 10.1073/pnas.2319574121. Epub 2024 Jul 18. Proc Natl Acad Sci U S A. 2024. PMID: 39024113 - Deletion of a core APC/C component reveals APC/C function in regulating neuronal USP1 levels and morphology.
Day JL, Tirard M, Brose N. Day JL, et al. Front Mol Neurosci. 2024 Jun 12;17:1352782. doi: 10.3389/fnmol.2024.1352782. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38932933 Free PMC article. - APC/C prevents non-canonical order of cyclin/CDK activity to maintain CDK4/6 inhibitor-induced arrest.
Mouery BL, Baker EM, Mills CA, Herring LE, Fleifel D, Cook JG. Mouery BL, et al. bioRxiv [Preprint]. 2023 Nov 9:2023.11.09.566394. doi: 10.1101/2023.11.09.566394. bioRxiv. 2023. PMID: 37986787 Free PMC article. Preprint. - Cell cycle exits and U-turns: Quiescence as multiple reversible forms of arrest.
Johnson MS, Cook JG. Johnson MS, et al. Fac Rev. 2023 Mar 8;12:5. doi: 10.12703/r/12-5. eCollection 2023. Fac Rev. 2023. PMID: 36923701 Free PMC article. Review.
References
- Amon A., Irniger, S., and Nasmyth, K. 1994. Closing the cell cycle circle in yeast: G2 cyclin proteolysis initiated at mitosis persists until the activation of G1 cyclins in the next cycle. Cell 77: 1037–1050. - PubMed
- Ben-Neriah Y. 2002. Regulatory functions of ubiquitination in the immune system. Nat. Immunol. 3: 20–26. - PubMed
- Conaway R.C., Brower, C.S., and Conaway, J.W. 2002. Emerging roles of ubiquitin in transcription regulation. Science 296: 1254–1258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous