Peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1alpha) regulates triglyceride metabolism by activation of the nuclear receptor FXR - PubMed (original) (raw)
. 2004 Jan 15;18(2):157-69.
doi: 10.1101/gad.1138104. Epub 2004 Jan 16.
Affiliations
- PMID: 14729567
- PMCID: PMC324422
- DOI: 10.1101/gad.1138104
Peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1alpha) regulates triglyceride metabolism by activation of the nuclear receptor FXR
Yanqiao Zhang et al. Genes Dev. 2004.
Abstract
Peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1alpha) has been shown to regulate adaptive thermogenesis and glucose metabolism. Here we show that PGC-1alpha regulates triglyceride metabolism through both farnesoid X receptor (FXR)-dependent and -independent pathways. PGC-1alpha increases FXR activity through two pathways: (1) it increases FXR mRNA levels by coactivation of PPARgamma and HNF4alpha to enhance FXR gene transcription; and (2) it interacts with the DNA-binding domain of FXR to enhance the transcription of FXR target genes. Ectopic expression of PGC-1alpha in murine primary hepatocytes reduces triglyceride secretion by a process that is dependent on the presence of FXR. Consistent with these in vitro studies, we demonstrate that fasting induces hepatic expression of PGC-1alpha and FXR and results in decreased plasma triglyceride levels in wild-type but not in FXR-null mice. Our data suggest that PGC-1alpha plays an important physiological role in maintaining energy homeostasis during fasting by decreasing triglyceride production/secretion while it increases fatty acid beta-oxidation to meet energy needs.
Figures
Figure 1.
Fasting and PGC-1α induce FXR mRNA levels. (A) Northern blot analysis of gene expression in fasted livers. FXR+/+ and FXR-/- mice (n = 4 or 5 per group) were fed either standard chow diet or fasted for 24 h. Total hepatic RNA was analyzed by Northern blot analysis using the indicated probes. (HL) Hepatic lipase. (B) Relative mRNA expression of FXRα1/α2 and FXRα3/α4 in fasted livers. Real-time PCR was used to identify the relative expression of FXRα1 + FXRα2 and FXRα3 + FXRα4 in the livers of mice either fed a chow diet or fasted for 24 h (n = 4 or 5 per group). The values are normalized to cyclophilin mRNA. (C) Ectopic expression of PGC-1α induces FXR expression. Murine primary hepatocytes were infected with adenovirus containing cDNA encoding either green fluorescent protein (GFP; Ad-GFP) or PGC-1α (Ad-PGC-1α) for 48 h, followed by treatment with either vehicle (DMSO) or the FXR ligand GW4064 (1 μM) for 24 h. Total RNA was isolated and mRNAs were identified by Northern blot assay. (D) PGC-1α differentially induces FXRα3/α4 expression in primary hepatocytes. FXR+/+ primary hepatocytes were infected with Ad-GFP or Ad-PGC-1α for 48 h. Total RNA was isolated and real-time PCR was used to analyze the relative expression of FXRα1 + α2 and FXRα3 + α4. (E) cAMP differentially induces FXRα3/α4 expression in primary hepatocytes. Primary hepatocytes were treated with or without 1 mM 8-bromo-cAMP (cAMP) for 24 h. Northern blot or real-time PCR was used to analyze the indicated gene expression. (*) p < 0.05; (**) p < 0.01.
Figure 2.
PGC-1α coactivates PPARγ and HNF4α to enhance FXR gene transcription. (A) PPARγ ligand induces FXR expression. Mouse primary hepatocytes were treated for 24 h with either vehicle (DMSO) or ligands for FXR (GW4064, 1 μM), PXR (PCN, 10 μM), LXR (T0901317, 1 μM), or PPARγ (GW7845, 1 μM). Total RNA was used for Northern blot assay. (B) Schematic representation of the 5′-end of the FXR gene showing the two FXR promoters and the nucleotides corresponding to the two DR-1 elements. FXRα1/α2 and FXRα3/α4 mRNAs are the products of transcription initiated from exon 1 or exon 3, respectively. Exon 1 is 26.6 kb upstream of exon 3. The sequences of DR-1 elements, and the incorporated mutations, are shown. The corresponding sequences of DR-1 elements in the promoters of the human FXR gene are also shown. (C,D) Activation of FXR promoters by PPARs. CV-1 cells were transiently transfected with either the FXR promoter-reporter constructs pGL3-FXRα1/α2-luc or pGL3-FXRα3/α4-luc together with PPARα, PPARδ, or PPARγ. The cells were treated with either vehicle or corresponding ligands (PPARα, 1 μM GW7847; PPARδ, 1 μM GW2433; PPARγ, 1 μM GW7845) for 36 h. Luciferase activity was analyzed and normalized with β-galactosidase activity. The values represent three independent experiments. (E,F) PPARγ/RXR binds to the DR-1 elements in both FXR promoters. Electrophoretic mobility-shift assays were performed using in vitro transcribed/translated receptors and radiolabeled FXRα1/α2 (E) or FXRα3/α4 (F) probes. For competition experiments, excess unlabeled competitor DNA, containing wild-type or mutant DR-1 sequences (B), was used at 20×, 100×, and 500×, respectively. (G,H) PGC-1α coactivates PPARγ to enhance FXR transcription via a DR-1 element. CV-1 cells were transfected with plasmids encoding PPARα, PPARγ, or PGC-1α together with either pGL3-FXRα1/α2-luc and pGL3-FXRα1/α2mut-luc (G) or pGL3-FXRα3/α4-luc and pGL3-FXRα3/α4mut-luc (H). Cells were incubated for 36 h in the presence or absence of specific PPAR ligands prior to determination of the luciferase activity. Values, normalized to β-galactosidase activity, are the means (±SE) of three experiments. (I,J) PGC-1α coactivates HNF4α to enhance FXR transcription via a DR-1 element. CV-1 cells were transfected with plasmids encoding PGC-1α or HNF4α together with FXR promoters as described in G and H. These values represent three independent experiments.
Figure 3.
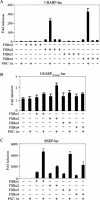
PGC-1α coactivates all FXR isoforms to enhance FXR target gene transcription. (_A_-C) HepG2 cells were transiently transfected in triplicate with the indicated I-BABP or BSEP promoter-reporter gene together with a specific FXR isoform and PGC-1α. The cells were treated with vehicle or GW4064 (1 μM). After 36 h the cells were lysed and the luciferase activity was determined after being normalized to β-galactosidase activity. These values represent the means (±SE) of three experiments.
Figure 4.
PGC-1α physically interacts with FXR in vitro and in cells. (A) Schematic representation of the domain structures of FXRα3 and the summary of the results to determine an interaction between PGC-1α (1-400) and various fragments of FXRα3. The domain structures of FXRα3 are shown (top). Interactions between PGC-1α (1-400) and the indicated fragments of FXRα3 are represented by + or - (right). (B) Mapping of the 5′ interaction domain of FXR with PGC-1α (1-400). 35S-labeled proteins corresponding to each of the four full-length FXR isoforms or 5′ deletions of FXRα3 or FXRα4 were incubated with GST-PGC-1α (1-400) in the presence of glutathione beads as described in Materials and Methods. The bound proteins were separated by SDS-PAGE and detected by autoradiography. (C) Mapping of the 3′ interaction domain of FXR with PGC-1α (1-400). The indicated N-terminal fragments of FXRα3 were in vitro labeled with 35S-methionine and used to determine the interaction with GST-PGC-1α (1-400), as described in B. (D) The DBD of FXR interacts directly with PGC-1α. 35S-labeled full-length PGC-1α protein was used to determine the interaction with the DBD or DBD plus the four amino acid insert (MYTG) of FXR. (E) Mapping the interaction domain of PGC-1α with FXR. 3′ deletions of PGC-1α with or without the mutation of the LXXLL motif were in vitro labeled with 35S-methionine and used to test interaction with the DBD of FXR. (F) FXR ligand has no effect on the interaction between FXR and PGC-1α. 35S-labeled, full-length FXRα1-4 were incubated with PGC-1α (1-400) in the presence or absence of GW4064 (1 μM). The assay was performed as described in B. (G) PGC-1α interacts with FXR in cells. CV-1 cells were transfected with CMX-Flag-PGC-1α and CMX-FXR plasmids, as indicated. After 48 h, whole-cell lysates were prepared and incubated with anti-Flag antibody. The immunoprecipitates were analyzed by SDS-PAGE/Western blot, using an anti-FXR antibody.
Figure 5.
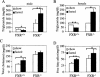
The change in plasma triglyceride levels in response to fasting is dependent on the FXR genotype. (A,B) Fasting decreases plasma triglyceride levels. Male (A) and female (B) FXR+/+ or FXR-/- mice (n = 4 or 5 per group) were either fed a chow diet or fasted for 24 h. Blood samples were collected and plasma triglyceride levels (mean ± SE) were determined. (C,D) Fasting increases total plasma cholesterol and plasma free fatty acids. Plasma total cholesterol (C) or free fatty acids (D; mean ± SE) are shown for the same mice. (*) p < 0.05; (**) p < 0.01.
Figure 6.
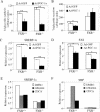
PGC-1α and FXR regulate triglyceride synthesis and secretion. (A) Ectopic expression of PGC-1α decreases triglyceride synthesis in primary hepatocytes. Wild-type and FXR-null primary hepatocytes were infected with adenovirus expressing GFP or PGC-1α. After 48 h, 14C-palmitic acid was added to the media. After an additional 2 h, the media and hepatocytes were separated and the radioactive triglyceride levels were determined, as described in Materials and Methods. Values are the means ± SE (n = 3). (B) Ectopic expression of PGC-1α decreases triglyceride secretion in an FXR-dependent manner. FXR+/+ or FXR-/- primary hepatocytes were infected with Ad-GFP or Ad-PGC-1α for 48 h and then incubated with 14C-palmitic acid for 2 h, as described in A. The radioactive triglyceride in the media was determined and the values were shown, mean ± SE (n = 3). (C,D) Ectopic expression of PGC-1α decreases SREBP-1c and FAS expression. Primary hepatocytes were infected with Ad-GFP or Ad-PGC-1α for 48 h. Real-time PCR was used to analyze the relative expression of SREBP-1c and fatty acid synthase (FAS). (E,F) Activation of FXR decreases SREBP-1c expression. Primary hepatocytes were treated with FXR ligand GW4064 (1 μM) or CDCA (100 μM) for 24 h. Real-time PCR was used to analyze the relative expression of SREBP-1c and SHP expression. (*) p < 0.05; (**) p < 0.01.
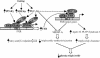
Figure 7.
Model for PGC-1α to activate FXR and regulate triglyceride metabolism. PGC-1α, PPARγ, and HNF4α mRNAs are induced after a prolonged fast. PGC-1α coactivates PPARγ and/or HNF4α bound to a DR-1 element in the FXR promoter, to induce FXR mRNA expression. In addition, PGC-1α interacts directly with FXR to enhance transcription of FXR target genes. Activation of FXR target genes by PGC-1α and FXR results in a decrease in SREBP-1c expression and in increased expression of genes involved in triglyceride metabolism and clearance. On the other hand, PGC-1α may also repress SREBP-1c expression in an FXR-independent manner. The decrease in SREBP-1c expression may reduce triglyceride synthesis/secretion and lower plasma triglyceride levels. The decrease in triglyceride synthesis in the liver may reduce storage of fatty acids and increase fatty acid β-oxidation to meet the normal energy demands during fasting.
Similar articles
- Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis.
Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. Rhee J, et al. Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4012-7. doi: 10.1073/pnas.0730870100. Epub 2003 Mar 21. Proc Natl Acad Sci U S A. 2003. PMID: 12651943 Free PMC article. - Ligand-dependent coactivation of the human bile acid receptor FXR by the peroxisome proliferator-activated receptor gamma coactivator-1alpha.
Savkur RS, Thomas JS, Bramlett KS, Gao Y, Michael LF, Burris TP. Savkur RS, et al. J Pharmacol Exp Ther. 2005 Jan;312(1):170-8. doi: 10.1124/jpet.104.072124. Epub 2004 Aug 25. J Pharmacol Exp Ther. 2005. PMID: 15329387 - Bile acid receptor agonist GW4064 regulates PPARγ coactivator-1α expression through estrogen receptor-related receptor α.
Dwivedi SK, Singh N, Kumari R, Mishra JS, Tripathi S, Banerjee P, Shah P, Kukshal V, Tyagi AM, Gaikwad AN, Chaturvedi RK, Mishra DP, Trivedi AK, Sanyal S, Chattopadhyay N, Ramachandran R, Siddiqi MI, Bandyopadhyay A, Arora A, Lundåsen T, Anakk SP, Moore DD, Sanyal S. Dwivedi SK, et al. Mol Endocrinol. 2011 Jun;25(6):922-32. doi: 10.1210/me.2010-0512. Epub 2011 Apr 14. Mol Endocrinol. 2011. PMID: 21493670 Free PMC article. - Regulation of energy metabolism by long-chain fatty acids.
Nakamura MT, Yudell BE, Loor JJ. Nakamura MT, et al. Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review. - FXR, a target for different diseases.
Wang YD, Chen WD, Huang W. Wang YD, et al. Histol Histopathol. 2008 May;23(5):621-7. doi: 10.14670/HH-23.621. Histol Histopathol. 2008. PMID: 18283647 Review.
Cited by
- Metabolic stress modulates Alzheimer's β-secretase gene transcription via SIRT1-PPARγ-PGC-1 in neurons.
Wang R, Li JJ, Diao S, Kwak YD, Liu L, Zhi L, Büeler H, Bhat NR, Williams RW, Park EA, Liao FF. Wang R, et al. Cell Metab. 2013 May 7;17(5):685-94. doi: 10.1016/j.cmet.2013.03.016. Cell Metab. 2013. PMID: 23663737 Free PMC article. - Dapagliflozin protects against nonalcoholic steatohepatitis in db/db mice.
Qiao P, Jia Y, Ma A, He J, Shao C, Li X, Wang S, Yang B, Zhou H. Qiao P, et al. Front Pharmacol. 2022 Aug 19;13:934136. doi: 10.3389/fphar.2022.934136. eCollection 2022. Front Pharmacol. 2022. PMID: 36059948 Free PMC article. - The role of nuclear receptors in the kidney in obesity and metabolic syndrome.
Tovar-Palacio C, Torres N, Diaz-Villaseñor A, Tovar AR. Tovar-Palacio C, et al. Genes Nutr. 2012 Oct;7(4):483-98. doi: 10.1007/s12263-012-0295-5. Epub 2012 Apr 25. Genes Nutr. 2012. PMID: 22532116 Free PMC article. - Organ-specific mediation of lifespan extension: more than a gut feeling?
Rera M, Azizi MJ, Walker DW. Rera M, et al. Ageing Res Rev. 2013 Jan;12(1):436-44. doi: 10.1016/j.arr.2012.05.003. Epub 2012 Jun 15. Ageing Res Rev. 2013. PMID: 22706186 Free PMC article. Review. - Controlling SIRT1 expression by microRNAs in health and metabolic disease.
Lee J, Kemper JK. Lee J, et al. Aging (Albany NY). 2010 Aug;2(8):527-34. doi: 10.18632/aging.100184. Aging (Albany NY). 2010. PMID: 20689156 Free PMC article.
References
- Ananthanarayanan M., Balasubramanian, N., Makishima, M., Mangelsdorf, D.J., and Suchy, F.J. 2001. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J. Biol. Chem. 276: 28857-28865. - PubMed
- Anisfeld A.M., Kast-Woelbern, H.R., Meyer, M.E., Jones, S.A., Zhang, Y., Williams, K.J., Willson, T., and Edwards, P.A. 2003. Syndecan-1 expression is regulated in an isoform-specific manner by the farnesoid-X receptor. J. Biol. Chem. 278: 20420-20428. - PubMed
- Castellani L.W., Wilcox, H.C., and Heimberg, M. 1991. Relationships between fatty acid synthesis and lipid secretion in the isolated perfused rat liver: Effects of hyperthyroidism, glucose and oleate. Biochim. Biophys. Acta 1086: 197-208. - PubMed
- Cullen P. 2000. Evidence that triglycerides are an independent coronary heart disease risk factor. Am. J. Cardiol. 86: 943-949. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases