Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis - PubMed (original) (raw)
Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis
Evelyn A Kurt-Jones et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Human neonates infected with herpes simplex virus 1 (HSV-1) develop one of three distinct patterns of infection: (i) infection limited to the skin, eye or mouth; (ii) infection of the CNS; or (iii) disseminated infection. The disseminated form usually involves the liver, adrenal gland, and lung, and resembles the clinical picture of bacterial sepsis. This spectrum of symptoms in HSV-1-infected neonates suggests that inflammatory cytokines play a significant role in the pathogenesis of the disease. Recent studies suggest that the Toll-like receptors (TLRs) may play an important role in the induction of inflammatory cytokines in response to viruses. TLRs are mammalian homologues of Toll, a Drosophila protein that is essential for host defense against infection. Engagement of TLRs by bacterial, viral, or fungal components leads to the production and release of cytokines and other antimicrobial products. Here, we demonstrate that TLR2 mediates the inflammatory cytokine response to HSV-1 by using both transfected cell lines and knockout mice. Studies of infected mice revealed that HSV-1 induced a blunted cytokine response in TLR2(-/-) mice. Brain levels of monocyte chemoattractant protein 1 chemokine were significantly lower in TLR2(-/-) mice than in either wild-type or TLR4(-/-) mice. TLR2(-/-) mice had reduced mortality compared with wild-type mice. The differences between TLR2(-/-) mice and both wild-type and TLR4(-/-) mice in the induction of monocyte chemoattractant protein 1, brain inflammation, or mortality could not be accounted for on the basis of virus levels. Thus, these studies suggest the TLR2-mediated cytokine response to HSV-1 is detrimental to the host.
Figures
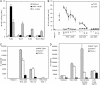
Fig. 1.
HSV activates cells through TLR2. (A) HEK293 cells expressing human TLR2, TLR3, or TLR4 ± MD2 were transfected with an NF-κB-driven firefly luciferase reporter plasmid and were stimulated for 6 h with HSV-1 (KOS strain) at a moi of 100 or with IL-1β (100 ng/ml) as a positive control. Luciferase activity was calculated in relative light units as a ratio of NF-κB-dependent firefly luciferase activity to NF-κB-independent Renilla luciferase activity. The results are shown as the mean ± SD of triplicate wells. Each cell line was tested in 3–10 independent experiments. (B) HEK293 cells expressing human TLR2 or TLR9 were challenged with HSV-1 KOS (a moi of 3–100), CpG DNA (0.1–3 μM), GpC control DNA (0.1–3 μM), or medium alone. NF-κB luciferase activity was measured as above. (C) Peritoneal exudates cells from wild-type, TLR2–/–, or TLR4–/– mice were stimulated with medium alone or with HSV-1 KOS at mois of 1, 10, and 100. IL-6 levels were measured in 16-h supernatants. The results are shown as the mean ± SD of duplicate wells. Each mouse strain was tested in at least three independent experiments. (D) Wild-type, TLR6–/–, or TLR2–/– peritoneal exudates cells were challenged with HSV-1 KOS (a moi of 100), Pam2CSK4 (100 ng/ml, a TLR2/TLR6 ligand), or LPS (10 ng/ml, a TLR4 ligand). IL-6 levels were measured as above.
Fig. 2.
Adult TLR2 knockout mice are resistant to lethal HSV-1 challenge. Groups of adult wild-type or TLR2–/– mice were challenged with 109 pfu of HSV-1 KOS virus i.p. Mice were observed for 1 week after challenge. Symptoms seen in mice included lethargy, ruffled fur, hindlimb paralysis, and seizures. All surviving mice were free of symptoms. (Eight mice per group, P ≤ 0.03, wild-type vs. TLR2–/– at day 4.)
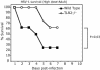
Fig. 3.
Neonatal TLR2 knockout mice are resistant to lethal HSV-1 challenge. Groups of 4-day-old mice were challenged with 104 pfu of HSV-1 KOS virus i.p. Mice were observed for 3 weeks after challenge. Symptoms seen in neonatal mice included spasmodic limb movement, hindlimb and total paralysis, and bloating. (Fourteen to 17 mice per group, P < 0.001, wild-type vs. TLR2–/– at day 6.)
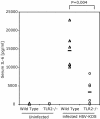
Fig. 4.
Wild-type mice produce higher levels of serum IL-6 cytokine than do TLR2 knockout mice in response to infection with HSV. Mice were infected with 109 pfu of HSV-KOS i.p. or uninfected. Blood was collected 24 h after infection, and serum IL-6 levels were determined by ELISA. (P = 0.004, wild-type vs. TLR2–/– at 24 h.)
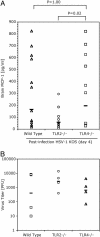
Fig. 5.
Wild-type mice produce higher levels of brain MCP-1 than do TLR2 knockout mice in response to infection with HSV. (A) Analysis of MCP-1 levels in the brains of HSV-1 KOS-infected mice. Wild-type, TLR2–/–, and TLR4–/– mice were infected with HSV-1 KOS i.p. (109 pfu of HSV i.p.). This dose was 50% lethal on day 4 in wild-type but not in TLR2–/– mice. Wild-type mice, but not TLR2–/– mice, were found to be moribund with brain hemorrhage at day 4. MCP-1 levels were measured in brain homogenates by ELISA. Levels in individual brains are shown. Geometric mean levels of MCP-1 are indicated by the bar. (B) Virus levels in brains on infected mice. Pfu of HSV-1 KOS in the brains of infected mice on day 4 after challenge with virus i.p. are shown. Levels in individual brains are shown. Geometric mean pfu is indicated by the bar.
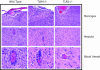
Fig. 6.
TLR2 knockout mice have less brain inflammation than do wild-type mice after HSV-1 challenge. Histopathology in the brains of HSV-1 KOS infected mice. Hematoxylin/eosin-stained section of cerebellum of mice 4 days postinfection with HSV i.p. (Top) Meninges with mononuclear cell infiltrates in wild-type and TLR4 knockouts. TLR2 knockout meninges are normal. (Middle) Cerebellum with mononuclear cell infiltrates and activated glial cells in wild-type and TLR4 knockouts. TLR2 knockout cerebellar tissue is normal. (Bottom) Blood vessels with accumulating mononuclear cells along the endothelial surface as well as perivascular cuffing in wild-type and TLR4 knockout brain. Blood vessels in TLR2 knockout brain are normal, with no evidence of inflammatory mononuclear cell accumulation.
Similar articles
- The role of toll-like receptors in herpes simplex infection in neonates.
Kurt-Jones EA, Belko J, Yu C, Newburger PE, Wang J, Chan M, Knipe DM, Finberg RW. Kurt-Jones EA, et al. J Infect Dis. 2005 Mar 1;191(5):746-8. doi: 10.1086/427339. Epub 2005 Jan 18. J Infect Dis. 2005. PMID: 15688290 - Toll-like receptor (TLR) 2 and TLR9 expressed in trigeminal ganglia are critical to viral control during herpes simplex virus 1 infection.
Lima GK, Zolini GP, Mansur DS, Freire Lima BH, Wischhoff U, Astigarraga RG, Dias MF, das Graças Almeida Silva M, Béla SR, do Valle Antonelli LR, Arantes RM, Gazzinelli RT, Báfica A, Kroon EG, Campos MA. Lima GK, et al. Am J Pathol. 2010 Nov;177(5):2433-45. doi: 10.2353/ajpath.2010.100121. Epub 2010 Sep 23. Am J Pathol. 2010. PMID: 20864677 Free PMC article. - Lethal encephalitis in myeloid differentiation factor 88-deficient mice infected with herpes simplex virus 1.
Mansur DS, Kroon EG, Nogueira ML, Arantes RM, Rodrigues SC, Akira S, Gazzinelli RT, Campos MA. Mansur DS, et al. Am J Pathol. 2005 May;166(5):1419-26. doi: 10.1016/S0002-9440(10)62359-0. Am J Pathol. 2005. PMID: 15855642 Free PMC article. - Impact of Toll-Like Receptors (TLRs) and TLR Signaling Proteins in Trigeminal Ganglia Impairing Herpes Simplex Virus 1 (HSV-1) Progression to Encephalitis: Insights from Mouse Models.
Campos MA, Zolini GP, Kroon EG. Campos MA, et al. Front Biosci (Landmark Ed). 2024 Mar 14;29(3):102. doi: 10.31083/j.fbl2903102. Front Biosci (Landmark Ed). 2024. PMID: 38538263 Review. - Viruses and Toll-like receptors.
Finberg RW, Kurt-Jones EA. Finberg RW, et al. Microbes Infect. 2004 Dec;6(15):1356-60. doi: 10.1016/j.micinf.2004.08.013. Microbes Infect. 2004. PMID: 15596120 Review.
Cited by
- A virological view of innate immune recognition.
Iwasaki A. Iwasaki A. Annu Rev Microbiol. 2012;66:177-96. doi: 10.1146/annurev-micro-092611-150203. Annu Rev Microbiol. 2012. PMID: 22994491 Free PMC article. Review. - Commensal bacteria lipoteichoic acid increases skin mast cell antimicrobial activity against vaccinia viruses.
Wang Z, MacLeod DT, Di Nardo A. Wang Z, et al. J Immunol. 2012 Aug 15;189(4):1551-8. doi: 10.4049/jimmunol.1200471. Epub 2012 Jul 6. J Immunol. 2012. PMID: 22772452 Free PMC article. - Innate lymphotoxin receptor mediated signaling promotes HSV-1 associated neuroinflammation and viral replication.
Liang Y, Yang K, Guo J, Wroblewska J, Fu YX, Peng H. Liang Y, et al. Sci Rep. 2015 May 20;5:10406. doi: 10.1038/srep10406. Sci Rep. 2015. PMID: 25993659 Free PMC article. - Novel drugs targeting Toll-like receptors for antiviral therapy.
Patel MC, Shirey KA, Pletneva LM, Boukhvalova MS, Garzino-Demo A, Vogel SN, Blanco JC. Patel MC, et al. Future Virol. 2014 Sep;9(9):811-829. doi: 10.2217/fvl.14.70. Future Virol. 2014. PMID: 25620999 Free PMC article. - Latent viral infections of the nervous system: role of the host immune response.
Lafon M. Lafon M. Rev Neurol (Paris). 2009 Dec;165(12):1039-44. doi: 10.1016/j.neurol.2009.09.010. Rev Neurol (Paris). 2009. PMID: 19906390 Free PMC article. Review.
References
- Corey, L. (2001) in Harrison's Principles of Internal Medicine, eds. Braunwald, E., Fauci, A. S., Kasper, D. L., Hauser, S. L., Longo, D. L. & Jameson, J. L. (McGraw–Hill, New York), pp. 1100–1106.
- Whitley, R. J. (2001) in Fields' Virology, eds. Knipe, D. M., Howley, P. M., Griffin, D. E., Lamb, R. A., Martin, M. A., Roizman, B. & Straus, S. E. (Lippincott, Williams & Wilkins, Philadelphia), 4th. Ed., pp. 2461–2509.
- Whitley, R., Arvin, A., Prober, C., Corey, L., Burchett, S., Plotkin, S., Starr, S., Jacobs, R., Powell, D., Nahmias, A., et al. (1991) N. Engl. J. Med. 324, 450–454. - PubMed
- Takeda, K., Kaisho, T. & Akira, S. (2003) Annu. Rev. Immunol. 21, 335–376. - PubMed
- Imler, J. L. & Zheng, L. (July 15, 2003) J. Leukocyte Biol., 10.1189/jlb.0403160.
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI051405/AI/NIAID NIH HHS/United States
- R01 AI020530/AI/NIAID NIH HHS/United States
- P01 NS35138/NS/NINDS NIH HHS/United States
- R01 AI39576/AI/NIAID NIH HHS/United States
- R01 AI051415/AI/NIAID NIH HHS/United States
- P01 NS035138/NS/NINDS NIH HHS/United States
- R01 GM63244/GM/NIGMS NIH HHS/United States
- R01 AI20530/AI/NIAID NIH HHS/United States
- R01 AI039576/AI/NIAID NIH HHS/United States
- R01 AI51415/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials