Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction - PubMed (original) (raw)
Comparative Study
Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction
Oliver Dewald et al. Am J Pathol. 2004 Feb.
Abstract
Large animal models have provided much of the descriptive data regarding the cellular and molecular events in myocardial infarction and repair. The availability of genetically altered mice may provide a valuable tool for specific cellular and molecular dissection of these processes. In this report we compare closed chest models of canine and mouse infarction/reperfusion qualitatively and quantitatively for temporal, cellular, and spatial differences. Much like the canine model, reperfused mouse hearts are associated with marked induction of endothelial adhesion molecules, cytokines, and chemokines. Reperfused mouse infarcts show accelerated replacement of cardiomyocytes by granulation tissue leading to a thin mature scar at 14 days, when the canine infarction is still cellular and evolving. Infarcted mouse hearts demonstrate a robust but transient postreperfusion inflammatory reaction, associated with a rapid up-regulation of interleukin-10 and transforming growth factor-beta. Unlike canine infarcts, infarcted mouse hearts show only transient macrophage infiltration and no significant mast cell accumulation. In correlation, the growth factor for macrophages, M-CSF, shows modest and transient up-regulation in the early days of reperfusion; and the obligate growth factor for mast cells, stem cell factor, SCF, is not induced. In summary, the postinfarction inflammatory response and resultant repair in the mouse heart shares many common characteristics with large mammalian species, but has distinct temporal and qualitative features. These important species-specific differences should be considered when interpreting findings derived from studies using genetically altered mice.
Figures
Figure 1
Progression of the healing process in reperfused canine (A–D) and murine (E–H) myocardial infarcts. Both canine (A) and murine (E) infarcts exhibit marked leukocyte infiltration after 24 hours of reperfusion. After 72 hours of reperfusion, dog infarcts show interstitial infiltration with inflammatory cells and fibroblasts (B) without significant cardiomyocyte replacement. In contrast, at the same time point, mouse infarcts show extensive cardiomyocyte replacement with granulation tissue (F). After 7 days of reperfusion the canine infarct is a highly cellular environment, whereas mouse infarcts exhibit areas of thinning. After 14 days of reperfusion the canine infarct still has a high cellular content (D). In contrast, the murine infarct shows thinned areas and large vascular structures (H). All sections are stained with hematoxylin and eosin (magnification, ×100).
Figure 2
Neutrophil immunohistochemistry in reperfused canine (A–C) and mouse (D–F) infarcts. Dog neutrophils were identified using the monoclonal anti-canine neutrophil antibody SG8H6 and mouse neutrophils were labeled with a rat anti-mouse neutrophil antibody. Canine neutrophils rapidly infiltrate the infarct after 24 hours of reperfusion (A), but their number decreases significantly after 72 hours of reperfusion (B). Few neutrophils are found in dog infarcts after 7 days of reperfusion (C, arrows). A large pericyte-poor vessel is noted (arrowhead), containing three intravascular neutrophils. A similar time course is noted in mouse infarcts: abundant neutrophils are found in the infarct after 24 hours of reperfusion (D), but decrease significantly after 72 hours (E). Neutrophils are rare in mouse infarcts after 7 days of reperfusion (F, arrows). Counterstained with eosin (magnification, ×400).
Figure 3
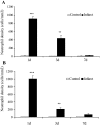
Quantitative analysis of neutrophil density in reperfused murine (A) and canine infarcts (B). Neutrophil infiltration in both mouse and dog infarcts is robust but transient, decreasing after 3 days of reperfusion. Neutrophil density in infarcts after 7 days of reperfusion is very low. ***, P < 0.001, **, P < 0.01.
Figure 4
Macrophage identification in reperfused canine (A–C) and mouse infarcts (D–F) using the monoclonal antibodies PM-2K (for the dog), and F4/80 (for the mouse). Canine macrophages progressively accumulate in the infarct after 24 hours (A, arrows) to 72 hours (B) of reperfusion, peaking after 7 days of reperfusion (C). In contrast, murine macrophages peak after 24 hours of reperfusion (D), remain abundant after 72 hours (E) and decrease significantly after 7 days of reperfusion (F, arrows). Sections counterstained with eosin (magnification, ×400).
Figure 5
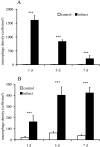
Quantitative analysis of macrophage density in reperfused murine (A, F4/80 staining) and canine infarcts (B, PM2K staining). Macrophage accumulation in reperfused mouse infarcts is robust but transient, decreasing after 7 days of reperfusion. In contrast, macrophage infiltration in dog infarcts is sustained, peaking after 7 days reperfusion. ***, P < 0.001.
Figure 6
α-smooth muscle actin (α-SMA) staining in reperfused canine (A–C) and murine infarcts (D–F). Dog infarcts show occasional α-SMA positive spindle-shaped cells (arrows) after 72 hours of reperfusion (A). These cells are phenotypically modulated fibroblasts, termed myofibroblasts. Myofibroblast accumulation increases dramatically after 7 days of reperfusion in the canine infarct (B, arrows). After 14 days of reperfusion α-SMA staining is predominantly localized in smooth muscle cells and pericytes coating maturing wound neovessels (C, arrowheads). In contrast, mouse infarcts exhibit a rapid and transient myofibroblast response. After 72 hours of reperfusion mouse infarcts show extensive myofibroblast infiltration (D, arrows). However, after 7 days of reperfusion myofibroblasts in the infarcted mouse heart significantly decrease and α-SMA staining is predominantly localized in pericyte-coated vessels (E, arrowheads) indicating rapid maturation of the infarct neovasculature. α-SMA immunoreactivity remains localized in vascular structures (arrowheads) after 14 days of reperfusion (F, arrowheads). G: Quantitative analysis of α-SMA staining in healing mouse infarcts. Infarct α-SMA % staining peaks after 3 days of reperfusion (***, P < 0.001 compared with non-infarcted heart) and decreases significantly after 7–14 days of reperfusion (#, P < 0.01 compared with % staining after 3 days of reperfusion).
Figure 7
Mast cells were identified in canine (A, B) and murine infarcts (D, E) using toluidine blue staining. Canine infarcts show significant mast cell accumulation after 3 days of reperfusion (A, arrows). Mast cell accumulation increases further after 7 days of reperfusion (B, arrows). In contrast, mouse infarcts have few mast cells in infarcted areas (arrow) after 3 (D), and 7 days of reperfusion (E). Diagrams show quantitative analysis of mast cell density in canine (C) and murine (F) infarcts. *, P < 0.05; ***, P < 0.001.
Figure 8
Vascular endothelial cells were identified in canine (A–C) and murine (D–F) infarcts using CD31 immunohistochemistry. The non-infarcted canine (A) and murine (D) heart has a rich microvascular network After 7 days of reperfusion canine infarcts have a high microvascular density (B) with a large number of capillaries, and occasional pericyte-poor dilated “mother vessels” (arrow). Mouse infarcts (i) at the same stage (E) exhibit a relatively low capillary density compared with non-infarcted areas (n) and have a significant number of dilated vascular structures (arrows). After 14 days of reperfusion (C) dog infarcts contain a large number of capillaries and a significant number of pericyte coated mature vessels (arrowhead), whereas mouse infarcts are thin containing predominantly enlarged vessels (arrows).
Figure 9
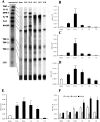
Cytokine expression in reperfused murine infarcts. A: Representative experiments demonstrate significant up-regulation of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α after 3 to 6 hours of reperfusion. The inhibitory cytokines IL-10 and TGF-β, and the matricellular protein osteopontin (OPN)−1, a marker of interstitial remodeling, are also upregulated in mouse infarcts. mRNA expression of IL-1β (B), IL-6 (C), and TNF-α (D) peaks after 6 hours of reperfusion but decreases significantly after 3 days of reperfusion. *, P < 0.05; ***, P < 0.001. mRNA E, F: Quantitative analysis of IL-10 (E) and TGF-β1, 2, and 3 (F) mRNA expression in reperfused murine infarcts. IL-10, TGF-β1, and TGF-β2 are transiently induced during reperfusion, in contrast to the later and more persistent up-regulation of TGF-β3. *, P < 0.05; **, P < 0.01; ***, P < 0.001, #, P < 0.01.
Figure 10
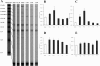
A: mRNA expression of the adhesion molecules ICAM-1 and E-selectin and the cytokine LIF is induced in mouse infarcts after 6 hours of reperfusion. In contrast expression of the adhesion molecule CD31/PECAM-1, and the cytokine receptors IL-10R and GM-CSF R do not change significantly after infarction. In addition, infarcted mouse hearts demonstrate a transient increase of M-CSF mRNA levels compared with sham-operated animals and show no SCF induction. B–E: Quantitative analysis of ICAM-1 (B), E-selectin (C), M-CSF (D) and SCF (E) mRNA levels in mouse infarcts. A modest transient induction of M-CSF (D) is found until 24 hours of reperfusion, associated with a transient macrophage accumulation in the infarcted mouse heart. Expression of SCF, a crucial factor for mast cell survival and growth did not show significant increase in murine infarcts (E). This finding may explain the absence of mast cell infiltration in healing mouse infarcts (Figure 7). **, P < 0.01; ***, P < 0.001.
Figure 11
A: Chemokine mRNA expression in reperfused mouse infarcts. MIP-1α, MIP-1β, MIP-2, and MCP-1 follow a similar pattern of transient up-regulation, peaking after 3–6 hours of reperfusion. In addition, IP-10 and its receptor CXCR3 are also induced in mouse infarcts. B–E: Quantitative analysis of MIP-1α (B), MIP-1β (C), MIP-2 (D), and MCP-1 (E) expression in canine infarcts. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Similar articles
- Extracellular matrix remodeling in canine and mouse myocardial infarcts.
Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, Frangogiannis NG. Dobaczewski M, et al. Cell Tissue Res. 2006 Jun;324(3):475-88. doi: 10.1007/s00441-005-0144-6. Epub 2006 Feb 22. Cell Tissue Res. 2006. PMID: 16496177 - Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts.
Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. Frangogiannis NG, et al. Circulation. 2005 Jun 7;111(22):2935-42. doi: 10.1161/CIRCULATIONAHA.104.510354. Epub 2005 May 31. Circulation. 2005. PMID: 15927970 - Stem cell-related cardiac gene expression early after murine myocardial infarction.
Vandervelde S, van Luyn MJ, Rozenbaum MH, Petersen AH, Tio RA, Harmsen MC. Vandervelde S, et al. Cardiovasc Res. 2007 Mar 1;73(4):783-93. doi: 10.1016/j.cardiores.2006.11.030. Epub 2006 Nov 29. Cardiovasc Res. 2007. PMID: 17208206 - Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion.
Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. Frangogiannis NG, et al. Circulation. 1998 Aug 18;98(7):687-98. doi: 10.1161/01.cir.98.7.687. Circulation. 1998. PMID: 9715862 - Inflammatory mechanisms in myocardial infarction.
Ren G, Dewald O, Frangogiannis NG. Ren G, et al. Curr Drug Targets Inflamm Allergy. 2003 Sep;2(3):242-56. doi: 10.2174/1568010033484098. Curr Drug Targets Inflamm Allergy. 2003. PMID: 14561159 Review.
Cited by
- Targeting the chemokines in cardiac repair.
Cavalera M, Frangogiannis NG. Cavalera M, et al. Curr Pharm Des. 2014;20(12):1971-9. doi: 10.2174/13816128113199990449. Curr Pharm Des. 2014. PMID: 23844733 Free PMC article. Review. - The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis.
Prabhu SD, Frangogiannis NG. Prabhu SD, et al. Circ Res. 2016 Jun 24;119(1):91-112. doi: 10.1161/CIRCRESAHA.116.303577. Circ Res. 2016. PMID: 27340270 Free PMC article. Review. - Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment.
Ip JE, Wu Y, Huang J, Zhang L, Pratt RE, Dzau VJ. Ip JE, et al. Mol Biol Cell. 2007 Aug;18(8):2873-82. doi: 10.1091/mbc.e07-02-0166. Epub 2007 May 16. Mol Biol Cell. 2007. PMID: 17507648 Free PMC article. - Intercellular model predicts mechanisms of inflammation-fibrosis coupling after myocardial infarction.
Chowkwale M, Lindsey ML, Saucerman JJ. Chowkwale M, et al. J Physiol. 2023 Jul;601(13):2635-2654. doi: 10.1113/JP283346. Epub 2022 Aug 8. J Physiol. 2023. PMID: 35862254 Free PMC article. - Characterizing the immune response to myocardial infarction in pigs.
Schnitter F, Stangl F, Noeske E, Bille M, Stadtmüller A, Vogt N, Sicklinger F, Leuschner F, Frey A, Schreiber L, Frantz S, Beyersdorf N, Ramos G, Gladow N, Hofmann U. Schnitter F, et al. Basic Res Cardiol. 2024 Jun;119(3):453-479. doi: 10.1007/s00395-024-01036-2. Epub 2024 Mar 15. Basic Res Cardiol. 2024. PMID: 38491291 Free PMC article.
References
- Jugdutt BI, Hutchins GM, Bulkley BH, Becker LC. Myocardial infarction in the conscious dog: three-dimensional mapping of infarct, collateral flow and region at risk. Circulation. 1979;60:1141–1150. - PubMed
- Michael LH, Lewis RM, Brandon TA, Entman ML. Cardiac lymph flow in conscious dogs. Am J Physiol. 1979;237:H311–317. - PubMed
- James JF, Hewett TE, Robbins J. Cardiac physiology in transgenic mice. Circ Res. 1998;82:407–415. - PubMed
- Franz WM, Mueller OJ, Hartong R, Frey N, Katus HA. Transgenic animal models: new avenues in cardiovascular physiology. J Mol Med. 1997;75:115–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials