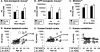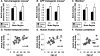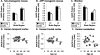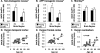Beta-secretase activity increases with aging in human, monkey, and mouse brain - PubMed (original) (raw)
Comparative Study
Beta-secretase activity increases with aging in human, monkey, and mouse brain
Hiroaki Fukumoto et al. Am J Pathol. 2004 Feb.
Abstract
Amyloid beta protein (A beta) accumulates in the brains of aging humans, amyloid precursor protein (APP) transgenic mouse lines, and rhesus monkeys. We tested the hypothesis that aging was associated with increased activity of the beta-site amyloid precursor protein cleaving enzyme (beta-secretase, BACE) in brain. We evaluated BACE activity, BACE protein, and formic acid-extractable A beta levels in cohorts of young (4 months old) and old (14 to 18 months old) nontransgenic mice (n = 16) and Tg2576 APP transgenic mice (n = 17), young (4.4 to 12.7 years old) and old (20.9 to 30.4 years old) rhesus monkeys (n = 17), and a wide age range (18 to 92 years old) of nondemented human brains (n = 25). Aging was associated with increased brain A beta levels in each cohort. Furthermore BACE activity increased significantly with age in mouse, monkey, and human brains, independent of brain region. BACE protein levels, however, were unchanged with age. BACE activity correlated with formic acid-extractable A beta levels in transgenic mouse, nontransgenic mouse, and human cortex, but not in monkey brain. These data suggest that an age-related increase of BACE activity contributes to the increased production and accumulation of brain A beta, and potentially predisposes to Alzheimer's disease in humans.
Figures
Figure 1
Formic acid-extractable Aβ measures in nontransgenic mouse (A), Tg2576 APP transgenic mouse (B), monkey (C), and human (D–F) brains with aging. In nontransgenic mice (A) and transgenic mice (B), Aβ levels were increased in 14- to 18-month-old mice (old) relative to 4-month-old mice (young) (*, analysis of variance; P < 0.001), reflecting significant increases in cortex (ctx) and cerebellum (cb), (†, posthoc _t_-test; P < 0.02). C: In rhesus monkey frontal (fr) and temporal (temp) cortices, there was a trend toward increased Aβ in the older monkeys (**, analysis of variance; P = 0.05). Aβ levels increased exponentially with age in human temporal cortex (D) and frontal cortex (E) (‡, P < 0.02) but not cerebellum (F).
Figure 2
BACE activity in nontransgenic mouse (A), Tg2576 APP transgenic mouse (B), monkey (C), and human (D–F) brains with aging. In nontransgenic mouse (A), transgenic mouse (B), and monkey (C) brains, BACE activity was increased in the old animals relative to the young animals (*, analysis of variance; P < 0.001), reflecting significant increases in each brain region (†, P < 0.05). BACE activity levels also increased with age in human temporal cortex (D), frontal cortex (E), and cerebellum (F) (‡, P < 0.03).
Figure 3
BACE protein levels in nontransgenic mouse (A), Tg2576 APP transgenic mouse (B), monkey (C), and human (D–F) brains with aging. There was no significant association of BACE protein levels with age in any of the cohorts.
Figure 4
Western blot of BACE (A) and pro-BACE (B) in human frontal cortices. There was no consistent change in the pattern of migration with aging.
Figure 5
The ratio of BACE activity to BACE protein (BACE act/prt) in nontransgenic mouse (A), Tg2576 APP transgenic mouse (B), monkey (C), and human (D–F) brains with aging. In nontransgenic mice (A) and transgenic mice (B), the BACE act/prt ratio was increased in the old mice relative to the young mice (*, analysis of variance; P < 0.001) reflecting significant increases in both cortex and cerebellum (†, posthoc; P < 0.01). In rhesus monkey (C), there was a trend toward increased BACE act/prt in the old monkeys relative to the young monkeys (**, analysis of variance; P = 0.07). In human brain, BACE act/prt increased with age in temporal cortex (D), frontal cortex (E), and cerebellum (F) (‡, P < 0.001).
Figure 6
BACE activity levels and Aβ levels in nontransgenic mouse (A), Tg2576 APP transgenic mouse (B), monkey (C), and human (D–F) brains. In nontransgenic mice (A) and transgenic mice (B), Aβ increased exponentially with increasing BACE activity levels in cortex and cerebellum (‡, P < 0.05). In monkey (C), there was no significant association between BACE activity levels and formic acid-extractable Aβ levels. In human brain, there were weak associations of BACE activity levels with Aβ40 levels in temporal cortex (D), and with Aβ40 and Aβ42 levels in frontal cortex (‡, P < 0.05) (E), but no association with Aβ levels in cerebellum (F).
Similar articles
- Neuronal and glial beta-secretase (BACE) protein expression in transgenic Tg2576 mice with amyloid plaque pathology.
Rossner S, Apelt J, Schliebs R, Perez-Polo JR, Bigl V. Rossner S, et al. J Neurosci Res. 2001 Jun 1;64(5):437-46. doi: 10.1002/jnr.1095. J Neurosci Res. 2001. PMID: 11391698 - Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer's disease patients.
Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H, Wong P, Price D, Shen Y. Li R, et al. Proc Natl Acad Sci U S A. 2004 Mar 9;101(10):3632-7. doi: 10.1073/pnas.0205689101. Epub 2004 Feb 20. Proc Natl Acad Sci U S A. 2004. PMID: 14978286 Free PMC article. - Age-dependent differential expression of BACE splice variants in brain regions of tg2576 mice.
Zohar O, Pick CG, Cavallaro S, Chapman J, Katzav A, Milman A, Alkon DL. Zohar O, et al. Neurobiol Aging. 2005 Aug-Sep;26(8):1167-75. doi: 10.1016/j.neurobiolaging.2004.10.005. Epub 2005 Jan 7. Neurobiol Aging. 2005. PMID: 15917100 - The β-secretase (BACE) inhibitor NB-360 in preclinical models: From amyloid-β reduction to downstream disease-relevant effects.
Neumann U, Machauer R, Shimshek DR. Neumann U, et al. Br J Pharmacol. 2019 Sep;176(18):3435-3446. doi: 10.1111/bph.14582. Epub 2019 Mar 10. Br J Pharmacol. 2019. PMID: 30657591 Free PMC article. Review. - The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential.
Vassar R, Kovacs DM, Yan R, Wong PC. Vassar R, et al. J Neurosci. 2009 Oct 14;29(41):12787-94. doi: 10.1523/JNEUROSCI.3657-09.2009. J Neurosci. 2009. PMID: 19828790 Free PMC article. Review.
Cited by
- Death by a thousand cuts in Alzheimer's disease: hypoxia--the prodrome.
Daulatzai MA. Daulatzai MA. Neurotox Res. 2013 Aug;24(2):216-43. doi: 10.1007/s12640-013-9379-2. Epub 2013 Feb 12. Neurotox Res. 2013. PMID: 23400634 Review. - Distinct patterns of APP processing in the CNS in autosomal-dominant and sporadic Alzheimer disease.
Pera M, Alcolea D, Sánchez-Valle R, Guardia-Laguarta C, Colom-Cadena M, Badiola N, Suárez-Calvet M, Lladó A, Barrera-Ocampo AA, Sepulveda-Falla D, Blesa R, Molinuevo JL, Clarimón J, Ferrer I, Gelpi E, Lleó A. Pera M, et al. Acta Neuropathol. 2013 Feb;125(2):201-13. doi: 10.1007/s00401-012-1062-9. Epub 2012 Dec 6. Acta Neuropathol. 2013. PMID: 23224319 Free PMC article. - SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10.
Donmez G, Wang D, Cohen DE, Guarente L. Donmez G, et al. Cell. 2010 Jul 23;142(2):320-32. doi: 10.1016/j.cell.2010.06.020. Cell. 2010. PMID: 20655472 Free PMC article. Retracted. - Sex-Specific Regulation of β-Secretase: A Novel Estrogen Response Element (ERE)-Dependent Mechanism in Alzheimer's Disease.
Cui J, Ait-Ghezala G, Sambamurti K, Gao F, Shen Y, Li R. Cui J, et al. J Neurosci. 2022 Feb 9;42(6):1154-1165. doi: 10.1523/JNEUROSCI.0864-20.2021. Epub 2021 Dec 13. J Neurosci. 2022. PMID: 34903570 Free PMC article. - BACE1 as a potential biomarker for Alzheimer's disease.
Decourt B, Sabbagh MN. Decourt B, et al. J Alzheimers Dis. 2011;24 Suppl 2(Suppl 2):53-9. doi: 10.3233/JAD-2011-110017. J Alzheimers Dis. 2011. PMID: 21403391 Free PMC article. Review.
References
- Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand. 1987;76:465–479. - PubMed
- Price DL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. - PubMed
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagoplan S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. - PubMed
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation and amyloid plaques in transgenic mice. Science. 1996;274:99–102. - PubMed
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA. 1997;94:13287–13292. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH/NS 31862/MH/NIMH NIH HHS/United States
- P01 AG015453/AG/NIA NIH HHS/United States
- P01 AG000001/AG/NIA NIH HHS/United States
- P51 RR000165/RR/NCRR NIH HHS/United States
- P50 AG005134/AG/NIA NIH HHS/United States
- AG15453/AG/NIA NIH HHS/United States
- AG00001/AG/NIA NIH HHS/United States
- AG05134/AG/NIA NIH HHS/United States
- RR00165/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases