Indirect sensing of insulin-induced hypoglycaemia by the carotid body in the rat - PubMed (original) (raw)
Indirect sensing of insulin-induced hypoglycaemia by the carotid body in the rat
I Bin-Jaliah et al. J Physiol. 2004.
Abstract
The most physiologically important sensors for systemic glucoregulation are located in extra-cranial sites. Recent evidence suggests that the carotid body may be one such site. We assessed rat carotid body afferent neural output in response to lowered glucose, indirectly by measurement of ventilation, and directly by recording single or few-fibre chemoafferent discharge, in vitro. Insulin (0.4 Ukg(-1)min(-1))-induced hypoglycaemia (blood glucose reduced by ca 50% to 3.4 +/- 0.1 mmoll(-1)) significantly increased spontaneous ventilation in sham-operated animals but not in bilateral carotid sinus nerve sectioned (CSNX) animals. In both groups, metabolic rate (measured as ) was almost doubled during hypoglycaemia. The ventilatory equivalent was unchanged in the sham group leading to a maintained control level of P(a, CO(2)), but was significantly reduced in the CSNX group, giving rise to an elevation of 6.0 +/- 1.3 mmHg in P(a, CO(2)). When pulmonary ventilation in sham animals was controlled and maintained, phrenic neural activity increased during hypoglycaemia and was associated with a significant increase in P(a, CO(2)) of 5.1 +/- 0.5 mmHg. Baseline chemoreceptor discharge frequency, recorded in vitro, was not affected, and did not increase when the superfusate [glucose] was lowered from 10 mm to 2 mm by substitution with sucrose: 0.40 +/- 0.20 Hz to 0.27 +/- 0.15 Hz, respectively (P > 0.20). We suggest therefore that any potential role of the carotid bodies in glucose homeostasis in vivo is mediated through its transduction of some other metabolically derived blood-borne factor rather than glucose per se and that this may also provide the link between exercise, metabolic rate and ventilation.
Figures
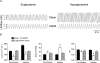
Figure 1. Insulin infusion decreases blood glucose concentration in sham and carotid sinus nerve-sectioned (CSNX) adult rats
Data points represent means ±
s.e.m.
of the blood glucose concentration in sham (n = 7) and CSNX (n = 7) animals. Insulin infusion, represented by the horizontal bar, began at time 0, was maintained for 120min and caused significant (P < 0.0001, ANOVA) and similar (_P_ > 0.15, ANOVA) amplitude falls from preinfusion levels of blood glucose in both groups of animals.
Figure 2. Effect of hypoglycaemia on spontaneous ventilation (_V̇_E), _P_a,CO2 and pH
A, representative traces of integrated tracheal airflow from a sham (above) and a CSNX (below) animal, taken 20min before insulin infusion (left) and at 60min after the infusion began (right). Inspiratory and expiratory flows were separately integrated and depicted in a single trace with + indicating inspiratory volume and – indicating expiratory volume. B, means ±
s.e.m.
of _V̇_E, _P_a,CO2 and pH in sham (_n_= 7), CSNX (n = 7) and euglycaemic clamped animals (n = 7) before (10–40min prior) and during (20–90min post) insulin infusion at 0.4Umin−1kg−1. * and † indicate significant difference (P < 0.05) from sham and CSNX preinfusion levels, respectively.
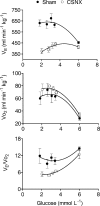
Figure 3. Changes in minute ventilation (_V̇_E), oxygen consumption (_V̇_O2) and ventilatory equivalent (_V̇_E/_V̇_O2) at varying blood glucose concentrations
Data points represent means ±
s.e.m.
in sham (n = 4) and CSNX (n = 4) animals. Glucose concentrations were measured at fixed times during the protocol period. During insulin-induced hypoglycaemia (blood glucose < 4 mmoll−1), _V̇_O2 increased significantly in sham and CSNX groups (P < 0.02, ANOVA) whilst _V̇_E increased only in sham (P < 0.002, ANOVA). _V̇_E/_V̇_O2 therefore decreased only in CSNX (P < 0.001, ANOVA) and remained unchanged in sham. Data are shown fitted by second order polynomials.
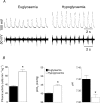
Figure 4. Phrenic nerve activity response to hypoglycaemia during controlled ventilation
Phrenic nerve discharge, blood gases and pH were recorded during insulin-induced hypoglycaemia in paralysed and artificially ventilated, sham animals (_n_= 4) in which the _P_a,CO2 was set at 39–41mmHg before infusion and maintained at this level of ventilation throughout the infusion period. A, example traces from a single experiment showing raw phrenic nerve discharge (lower trace) and integrated discharge (100 ms time constant; upper trace) during euglycaemia (6.1mmoll−1) and hypoglycaemia (3.2mmoll−1). B, phrenic nerve discharge, _P_a, CO2 and pH data expressed as means ±
s.e.m.
* indicates significant difference (P < 0.05, Student's t test) from euglycaemic levels.
Figure 5. In vitro carotid sinus nerve afferent discharge is unaffected by decreasing glucose
Single-fibre chemoafferent discharge recorded from one animal during control (10m
m
glucose) and low glucose superfusion (2m
m
glucose; bar). Discharge was binned into 20-s periods and frequency calculated as impulses s−1 (Hz). Discharge did not change significantly from control during the period of low glucose superfusion. On the right are shown five superimposed afferent action potentials from this recording. The vertical scale bar is 100 mV, horizontal scale bar 0.4 ms.
Similar articles
- Carbon dioxide sensitivity during hypoglycaemia-induced, elevated metabolism in the anaesthetized rat.
Bin-Jaliah I, Maskell PD, Kumar P. Bin-Jaliah I, et al. J Physiol. 2005 Mar 15;563(Pt 3):883-93. doi: 10.1113/jphysiol.2004.080085. Epub 2005 Jan 20. J Physiol. 2005. PMID: 15661819 Free PMC article. - Adrenaline release evokes hyperpnoea and an increase in ventilatory CO2 sensitivity during hypoglycaemia: a role for the carotid body.
Thompson EL, Ray CJ, Holmes AP, Pye RL, Wyatt CN, Coney AM, Kumar P. Thompson EL, et al. J Physiol. 2016 Aug 1;594(15):4439-52. doi: 10.1113/JP272191. Epub 2016 May 5. J Physiol. 2016. PMID: 27027261 Free PMC article. - Intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats.
Bavis RW, Mitchell GS. Bavis RW, et al. J Appl Physiol (1985). 2003 Jan;94(1):399-409. doi: 10.1152/japplphysiol.00374.2002. Epub 2002 Jul 12. J Appl Physiol (1985). 2003. PMID: 12391138 - Carotid chemoreflex. Neural pathways and transmitters.
Sapru HN. Sapru HN. Adv Exp Med Biol. 1996;410:357-64. Adv Exp Med Biol. 1996. PMID: 9030325 Review. - Adrenaline activation of the carotid body: Key to CO2 and pH homeostasis in hypoglycaemia and potential pathological implications in cardiovascular disease.
Holmes AP, Ray CJ, Thompson EL, Alshehri Z, Coney AM, Kumar P. Holmes AP, et al. Respir Physiol Neurobiol. 2019 Jul;265:92-99. doi: 10.1016/j.resp.2018.05.008. Epub 2018 May 25. Respir Physiol Neurobiol. 2019. PMID: 29807139 Review.
Cited by
- TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing.
Buckler KJ. Buckler KJ. Pflugers Arch. 2015 May;467(5):1013-25. doi: 10.1007/s00424-015-1689-1. Epub 2015 Jan 28. Pflugers Arch. 2015. PMID: 25623783 Free PMC article. Review. - Insulin and sympathoexcitation: it is not all in your head.
Joyner MJ, Limberg JK. Joyner MJ, et al. Diabetes. 2013 Aug;62(8):2654-5. doi: 10.2337/db13-0613. Diabetes. 2013. PMID: 23881194 Free PMC article. No abstract available. - The Bidirectional Relationship Between Obstructive Sleep Apnea and Metabolic Disease.
Framnes SN, Arble DM. Framnes SN, et al. Front Endocrinol (Lausanne). 2018 Aug 6;9:440. doi: 10.3389/fendo.2018.00440. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30127766 Free PMC article. Review. - Peripheral and central glucose sensing in hypoglycemic detection.
Donovan CM, Watts AG. Donovan CM, et al. Physiology (Bethesda). 2014 Sep;29(5):314-24. doi: 10.1152/physiol.00069.2013. Physiology (Bethesda). 2014. PMID: 25180261 Free PMC article. Review. - Role of K₂p channels in stimulus-secretion coupling.
Kim D, Kang D. Kim D, et al. Pflugers Arch. 2015 May;467(5):1001-11. doi: 10.1007/s00424-014-1663-3. Epub 2014 Dec 6. Pflugers Arch. 2015. PMID: 25476848 Free PMC article. Review.
References
- Alfaro V, Palacios L. Components of the blood acid-base disturbance that accompanies urethane anaesthesia in rats during normothermia and hypothermia. Clin Exp Pharmacol Physiol. 1997;24:498–502. - PubMed
- Almaraz L, Obeso A, Gonzalez C. Metabolic dissociation of carotid body chemoreceptors responses to different types of stimulation: preliminary findings. In: Pallot DJ, editor. The Peripheral Arterial Chemoreceptors. London: Croom-Helm; 1984. pp. 141–151.
- Alvarez-Buylla R, de Alvarez-Buylla ER. Carotid sinus receptors participate in glucose homeostasis. Respir Physiol. 1988;72:347–359. - PubMed
- Alvarez-Buylla R, de Alvarez-Buylla ER. Changes in blood glucose concentration in the carotid body-sinus modify brain glucose retention. Brain Res. 1994;654:167–170. - PubMed
- Bamford OS, Carroll JL. Dynamic ventilatory responses in rats: normal development and effects of prenatal nicotine exposure. Respir Physiol. 1999;117:29–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical