Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors - PubMed (original) (raw)
Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors
Feras I Hawari et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Soluble tumor necrosis factor receptors (TNFRs) are important modulators of TNF bioactivity. Proteolytic cleavage of the 28-kDa ectodomain of TNFR1 has been recognized as the mechanism by which soluble TNFR is shed. We now describe the release of exosome-like vesicles as a mechanism for the generation of soluble, full-length 55-kDa TNFR1. We found unexpectedly that the predominant form of soluble TNFR1 in human serum and lung epithelial lining fluid is a full-length 55-kDa protein. Furthermore, supernatants from human vascular endothelial cells contain only full-length 55-kDa TNFR1 that can be sedimented by high-speed centrifugation, floated on sucrose gradients at a density of 1.1 g/ml, and associated with vesicles that range in diameter from 20 nm to 50 nm. We conclude that the release of TNFR1 exosome-like vesicles represents a previously unrecognized mechanism by which constitutive production of soluble cytokine receptors may be regulated, independent of ectodomain cleavage by receptor sheddases.
Figures
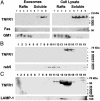
Fig. 1.
(A) Full length 55-kDa is the predominant form of soluble TNFR1 present in human serum and lung epithelial lining fluid. Samples of proteins (60 μg) of serum and BALF from healthy research volunteers, or medium from HUVEC, were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with a murine IgG2b monoclonal antibody (H-5) against the extracellular domain of human TNFR1. A recombinant human TNFR1 extracellular domain (rhTNFR1) is shown as a positive control. (B) Characterization of HUVEC-derived soluble TNFR1 as the full-length 55-kDa receptor by sequential centrifugation. Samples of proteins (60 μg) from the pellets [200 × g (P1), 500 × g (P2), 1,200 × g (P3), 10,000 × g (P4) or 100,000 × g (P5)], and the 100,000 × g supernatant (S5) from HUVEC medium, were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with the H-5 anti-TNFR1 antibody. Also shown are total cellular (T) and membrane (M) proteins. Nitrocellulose membranes were stripped and reacted with antibodies against rab5 and LAMP-1. (C) Characterization of HUVEC-derived soluble TNFR1. Sequential centrifugations were performed as described in B. Samples of proteins (60 μg) from the pellets [100,000 × g (P5), 175,000 × g (P6)] and supernatants [100,000 × g (S5), 175,000 × g (S6)] from HUVEC medium were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with the H-5 anti-TNFR1 antibody. These immunoblots are representative of three experiments demonstrating similar results. (D) Characterization of HUVEC-derived soluble TNFR1. Samples of proteins (40 μg) from HUVEC lysates (T) and medium that had been precleared of cells and debris by sequential centrifugation at 1,200 × g and 10,000 × g (S4) were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with the H-5 anti-TNFR1 antibody. C-S4 denotes a 30-fold concentration of the S4 supernatant. (E) Confirmation of TNFR1 in HUVEC-derived secreted vesicles. Samples of proteins (60 μg) from the 175,000 × g pellet (P6) were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with the H-5 (EC 1) and the clone 16805.21 (EC 2) murine monoclonal antibodies directed against the TNFR1 extracellular domain and the C-20 goat polyclonal antibody directed against the TNFR1 intracytoplasmic domain. (F) Characterization of the TNFR1 signaling complex I in HUVEC-derived exosome-like vesicles. Samples of proteins (60 μg) from whole cell lysates (T) and the 175,000 × g pellet (P6) were separated by SDS/PAGE, transferred to nitrocellulose and reacted with antibodies against SODD, TRADD, RIP, and TRAF2.
Fig. 2.
Characterization of HUVEC-derived exosome-associated TNFR1 by density gradient centrifugation. (A) HUVEC-derived TNFR1 exosomes are not comprised of lipid raft microdomains. Vesicles (2 mg) were isolated from HUVEC medium, suspended in 1% Triton X-100 at 4°C, and subjected to sucrose gradient centrifugation to isolate lipid raft microdomains. HUVEC were also lysed in 1% Triton X-100 at 4°C, and lipid raft microdomains were similarly isolated. Fractions (1 ml) were collected from the top, and 60 μg of protein from each fraction were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with antibodies against TNFR1. Nitrocellulose membranes were stripped and reacted with an antibody against Fas, a marker of Triton X-100 soluble fractions. The distribution of GM1, a marker of lipid raft microdomains, was detected by dot blotting 3 μl of each fraction onto nitrocellulose membranes and detected by using cholera toxin B subunit–peroxidase conjugate (CTxHRP). (B) Rate zonal centrifugation through continuous sucrose gradients. Vesicles in HUVEC medium were sedimented by centrifugation at 175,000 × g for 16 h (4°C) and resuspended in medium. A sample containing 2 mg of vesicles was layered on top of a continuous sucrose gradient (0.2–2.5 M in 20 mM Tris, pH 8) and centrifuged at 200,000 × g for 16 h. Fractions (0.5 ml) were collected from the bottom, and samples of proteins (60 μg) were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with antibodies against TNFR1. Nitrocellulose membranes were stripped and reprobed with antibodies against rab5 (B) or LAMP-1 (C). Lane numbers correspond to the fractions collected. Molecular mass markers are shown on the right of each immunoblot. These immunoblots are representative of duplicate experiments demonstrating similar results.
Fig. 3.
Localization of TNFR1 to exosome-like vesicles by immunoelectron microscopy. Samples of bronchoalveolar lavage fluid (A_–_C) and HUVEC medium (D and E) were centrifuged at 500 × g for 10 min and 10,000 × g for 30 min to remove cellular debris. Samples were then concentrated by using a Centriprep filter with a 3,000-kDa exclusion, applied to Formvar-carbon-coated nickel electron microscopy grids, and immunostained with the antibodies against TNFR1. As shown by ImmunoGold labeling, TNFR1 was localized to small, irregularly shaped vesicles of 20–50 nm in diameter (arrows), consistent with exosome-like vesicles in both bronchoalveolar lavage fluid (A and B) and HUVEC-conditioned media (D). No ImmunoGold labeling was detected when ImmunoGold labeling was performed with an IgG2b isotype control antibody (C and E). These images are representative of duplicate experiments demonstrating similar results.
Fig. 4.
(A) Zinc metalloprotease activity is required for soluble TNFR1 release. HUVEC were treated with 0.1% DMSO, 25 μM Z-VAD-FMK, or 50 μM TAPI-2 for 24 h, and concentrations of sTNFR1 present in culture supernatants were determined by ELISA (n = 6). *, P < 0.05 vs. DMSO-treated cells. (B) Effect of TAPI-2 on TNFR1 exosome-like vesicle release. HUVEC were treated with (+)(C and D) or without (–) (A and B) 50 μM TAPI-2 for 24 h, and medium was subjected to sequential centrifugation. Samples of proteins (60 μg), in duplicate, from whole cell lysates (T) and 175,000 × g (P6) pellets were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with antibodies against TNFR1. The average relative mean density of the TAPI-2-treated whole cell lysates was 1.56 as compared with 1.0 for the cells treated with medium alone, whereas the relative mean density of the TAPI-2-treated exosomes was 1.0 as compared with 1.75 for exosomes treated with medium alone. (C) HUVEC-derived exosomes bind TNF-α. Exosomes were isolated from HUVEC medium as described, resuspended in medium, and incubated with 1 ng of recombinant human TNF-α for 16 h at 4°C. Exosomes were recovered by centrifugation at 175,000 × g for 16 h at 4°C, separated by SDS/PAGE, transferred to nitrocellulose, and reacted with antibodies against TNF-α. (D) Binding of rhTNF-α to HUVEC-derived exosomes. TNFR1 exosomes (100 μg) were immunoprecipitated with a murine monoclonal antibody directed against the TNFR1 extracellular domain (clone 16805.21) and incubated with rhTNF-α, and the quantity of bound rhTNF-α, in duplicate samples, was measured by ELISA.
Fig. 5.
(A) Characterization of soluble TNFR1 from human lung epithelial lining fluid. Samples of proteins (60 μg) from the sequential pellets of BALF, as described in Fig. 2, were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with antibodies against TNFR1. Nitrocellulose membranes were stripped and reacted with antibodies against rab5. This blot is representative of four of the seven normal volunteers. (B) Sequential centrifugation of BALF. Duplicate samples of proteins (60 μg) from the 100,000 × g (P5) pellet were separated by SDS/PAGE, transferred to nitrocellulose, and reacted with antibodies against TNFR1. This blot is representative of three of the seven normal volunteers.
References
- Chen, G. & Goeddel, D. V. (2002) Science 296, 1634–1635. - PubMed
- Jiang, Y., Woronicz, J. D., Liu, W. & Goeddel, D. V. (1999) Science 283, 543–546. - PubMed
- Engelmann, H., Aderka, D., Rubinstein, M., Rotman, D. & Wallach, D. (1989) J. Biol. Chem. 264, 11974–11980. - PubMed
- Olsson, I., Lantz, M., Nilsson, E., Peetre, C., Thysell, H., Grubb, A. & Adolf, G. (1989) Eur. J. Haematol. 42, 270–275. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources