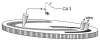Homeostatic plasticity in hippocampal slice cultures involves changes in voltage-gated Na+ channel expression - PubMed (original) (raw)
Homeostatic plasticity in hippocampal slice cultures involves changes in voltage-gated Na+ channel expression
Caitlin O Aptowicz et al. Brain Res. 2004.
Abstract
Neurons preserve stable electrophysiological properties despite ongoing changes in morphology and connectivity throughout their lifetime. This dynamic compensatory adjustment, termed 'homeostatic plasticity', may be a fundamental means by which the brain normalizes its excitability, and is possibly altered in disease states such as epilepsy. Despite this significance, the cellular mechanisms of homeostatic plasticity are incompletely understood. Using field potential analyses, we observed a compensatory enhancement of neural excitability after 48 h of activity deprivation via tetrodotoxin (TTX) in hippocampal slice cultures. Because activity deprivation can enhance voltage-gated sodium channel (VGSC) currents, we used Western blot analyses to probe for these channels in control and activity-deprived slice cultures. A significant upregulation of VGSCs expression was evident after activity deprivation. Furthermore, immunohistochemistry revealed this upregulation to occur along primarily pyramidal cell dendrites. Western blot analyses of cultures after 1 day of recovery from activity deprivation showed that VGSC levels returned to control levels, indicating that multiple molecular mechanisms contribute to enhanced excitability. Because of their longevity and in vivo-like cytoarchitecture, we conclude that slice cultures may be highly useful for investigating homeostatic plasticity. Furthermore, we demonstrate that enhanced excitability involves changes in channel expression with a targeted localization likely profound transform the integrative capacities of hippocampal pyramidal cells and their dendrites.
Figures
Fig. 1
Schematic of electrophysiological recording paradigm. Bipolar stimulating electrode (stim) in the DG was used to evoke population spike in the CA3 pyramidal layer. The latter was recorded by a microelectrode placed in the interstitial space that recorded the typical negative (population spike) then positive (fEPSP), marked by large arrow and small arrow, respectively.
Fig. 2
TTX treatment reversibly increases excitability of CA3 neurons in hippocampal slices cultures without cell death. (A) Increased excitability is evident in extracellularly recorded responses from area CA3 to a single stimulatory pulse in dentate gyrus (DG), recorded after allowing cultures to rest in the electrophysiological setup for 30 min. Upper trace shows typical response in controls and lower trace shows typical multiple spikes produced by same stimulus in activity deprived cultures. Vertical calibration represents 2 mV and the horizontal calibration represents 50 ms. (B) Interstimulus interval (ISI) affects spike number with the most robust response occurring at the longest ISI. Each bar represents average (n = 7) number of spikes evoked. Cultures were always tested for their responses to stimulation in the same order (ISI of 10, 5, 3, and 1 min) and were then retested for their response to stimulation at an ISI of 10 min (last bar). All responses were significantly (P ≤0.001) greater than those evoked in control cultures at each stimulation interval tested, as indicated by asterisk. We rejected spikes less than 2 mV in amplitude when counting. (C) Enhanced excitability is completely reversed for all ISIs by 3 days after 48 h of activity deprivation. Four bars grouped in each day represent, from left to right, the response to stimulation at intervals of 10, 5, 3, and 1 min. (D) Sytox™ measurement of cell death in slice cultures. Top panel is representative of control cultures (n = 6) exhibiting only bright green stained cells indicative of normal cell death in DG (emphasized here by white elliptical line and arrow; with arrow alone used for similar purposes in two lower panels). Middle panel is representative of both TTX-exposed slice cultures and cultures exposed to TTX and returned to normal media for up to 7 days showing no increased cell death compared with controls. For positive control, the bottom panel shows robust cell death in CA1 24 h after 30 min of incubation in media containing 10 μM NMDA.
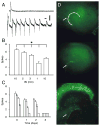
Fig. 3
VGSCs are upregulated in activity-deprived slice cultures as revealed by Western blot and immunohistochemical analyses. (A) Representative Western blot of VGSC expression illustrates an increase in channel expression from control (CTRL) to 48 h activity-deprived (TTX) cultures. (B) Quantification of increased VGSC expression by densitometric analysis shows a significant (*: P < 0.001) increase in VGSC expression in TTX treated cultures compared with controls (n = 5 per group). All measurements were normalized to the control average. (C) Representative low-power images of VGSC immunostaining in control (upper) versus activity-deprived (lower) cultures. Images show CA3 to left and dentate gyrus (DG) to right. TTX treatment triggered increased immunostaining that was concentrated to the stratum oriens of CA3 that consists mainly of the basilar dendrites from pyramidal neurons (leftmost aspect of images). In addition, increased immunostaining also was evident in the plexiform layer of the DG and to a lesser extent in the stratum lucidum of CA3, two other hippocampal areas enriched with dendrites. (D) No significant difference in VGSC expression was seen between TTX-treated and respective control cultures 1, 2 and 3 days after exposure to TTX (n = 3). Representative Western blot of VGSC expression in control (CTRL) and activity-deprived (TTX) cultures recovered for 1 day are shown. Analogous results were seen for cultures recovered 2 and 3 days (data not shown). Calibration bar in C is 100 μm.
Similar articles
- NMDA receptors and L-type voltage-gated Ca²⁺ channels mediate the expression of bidirectional homeostatic intrinsic plasticity in cultured hippocampal neurons.
Lee KY, Chung HJ. Lee KY, et al. Neuroscience. 2014 Sep 26;277:610-23. doi: 10.1016/j.neuroscience.2014.07.038. Epub 2014 Jul 31. Neuroscience. 2014. PMID: 25086314 - Activity deprivation leads to seizures in hippocampal slice cultures: is epilepsy the consequence of homeostatic plasticity?
Trasande CA, Ramirez JM. Trasande CA, et al. J Clin Neurophysiol. 2007 Apr;24(2):154-64. doi: 10.1097/WNP.0b013e318033787f. J Clin Neurophysiol. 2007. PMID: 17414971 - Plasticity of both excitatory and inhibitory synapses is associated with seizures induced by removal of chronic blockade of activity in cultured hippocampus.
Bausch SB, He S, Petrova Y, Wang XM, McNamara JO. Bausch SB, et al. J Neurophysiol. 2006 Oct;96(4):2151-67. doi: 10.1152/jn.00355.2006. Epub 2006 Jun 21. J Neurophysiol. 2006. PMID: 16790597 - Homeostatic plasticity in neural development.
Tien NW, Kerschensteiner D. Tien NW, et al. Neural Dev. 2018 Jun 1;13(1):9. doi: 10.1186/s13064-018-0105-x. Neural Dev. 2018. PMID: 29855353 Free PMC article. Review. - The other half of Hebb: K+ channels and the regulation of neuronal excitability in the hippocampus.
Schrader LA, Anderson AE, Varga AW, Levy M, Sweatt JD. Schrader LA, et al. Mol Neurobiol. 2002 Feb;25(1):51-66. doi: 10.1385/MN:25:1:051. Mol Neurobiol. 2002. PMID: 11890457 Review.
Cited by
- Homeostatic regulation of extracellular signal-regulated kinase 1/2 activity and axonal Kv7.3 expression by prolonged blockade of hippocampal neuronal activity.
Baculis BC, Kesavan H, Weiss AC, Kim EH, Tracy GC, Ouyang W, Tsai NP, Chung HJ. Baculis BC, et al. Front Cell Neurosci. 2022 Jul 28;16:838419. doi: 10.3389/fncel.2022.838419. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35966206 Free PMC article. - Development and plasticity of spontaneous activity and Up states in cortical organotypic slices.
Johnson HA, Buonomano DV. Johnson HA, et al. J Neurosci. 2007 May 30;27(22):5915-25. doi: 10.1523/JNEUROSCI.0447-07.2007. J Neurosci. 2007. PMID: 17537962 Free PMC article. - A theory of rate coding control by intrinsic plasticity effects.
Naudé J, Paz JT, Berry H, Delord B. Naudé J, et al. PLoS Comput Biol. 2012 Jan;8(1):e1002349. doi: 10.1371/journal.pcbi.1002349. Epub 2012 Jan 19. PLoS Comput Biol. 2012. PMID: 22275858 Free PMC article. - Silencing-induced metaplasticity in hippocampal cultured neurons.
Sokolova IV, Mody I. Sokolova IV, et al. J Neurophysiol. 2008 Aug;100(2):690-7. doi: 10.1152/jn.90378.2008. Epub 2008 May 28. J Neurophysiol. 2008. PMID: 18509070 Free PMC article. - Homeostatic scaling of excitability in recurrent neural networks.
Remme MW, Wadman WJ. Remme MW, et al. PLoS Comput Biol. 2012;8(5):e1002494. doi: 10.1371/journal.pcbi.1002494. Epub 2012 May 3. PLoS Comput Biol. 2012. PMID: 22570604 Free PMC article.
References
- Bahr BA. Long-term hippocampal slices: a model system for investigating synaptic mechanisms and pathological processes. J Neurosci Res. 1995;42:294–305. - PubMed
- Bahr BA, Kessler M, Rivera S, Vanderklish PW, Hall RA, Mutneja MS, Gall C, Hoffman KB. Stable maintenance of glutamate receptors and other synaptic components in long-term hippocampal slices. Hippocampus. 1995;5:425–439. - PubMed
- Beal MF. Role of excitotoxicity in human neurological disease. Curr Opin Neurobiol. 1992;5:657–662. - PubMed
- Bien A, Seidenbecher CI, Bockers TM, Sabel BA, Kreutz MR. Apoptotic versus necrotic characteristics of retinal ganglion cell death after partial optic nerve injury. J Neurotrauma. 1999;16:153–163. - PubMed
- Brodie C, Sampson SR. Effects of ethanol on voltage-sensitive Na-channels in cultured skeletal muscle: up-regulation as a result of chronic treatment. J Pharmacol Exp Ther. 1990;255:1195–1201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources