Absence of dystrophin in mice reduces NO-dependent vascular function and vascular density: total recovery after a treatment with the aminoglycoside gentamicin - PubMed (original) (raw)
Comparative Study
Absence of dystrophin in mice reduces NO-dependent vascular function and vascular density: total recovery after a treatment with the aminoglycoside gentamicin
Laurent Loufrani et al. Arterioscler Thromb Vasc Biol. 2004 Apr.
Abstract
Objective: Mutations in the dystrophin gene causing Duchenne's muscular dystrophy (DMD) lead to premature stop codons. In mice lacking dystrophin (mdx mice), a model for DMD, these mutations can be suppressed by aminoglycosides such as gentamicin. Dystrophin plays a role in flow (shear stress)-mediated endothelium-dependent dilation (FMD) in arteries. We investigated the effect of gentamicin on vascular contractile and dilatory functions, vascular structure, and density in mdx mice.
Methods and results: Isolated mice carotid and mesenteric resistance arteries were mounted in arteriographs allowing continuous diameter measurements. Mdx mice showed lower nitric oxide (NO)-dependent FMD and endothelial NO synthase (eNOS) expression as well as decreased vascular density in gracilis and cardiac muscles compared with control mice. Treatment with gentamycin restored these parameters. In contrast, smooth muscle-dependent contractions as well as endothelium-dependent or -independent dilation were not affected by dystrophin deficiency or by gentamicin treatment.
Conclusions: Dystrophin deficiency induces a selective defect in flow-dependent mechanotransduction, thus attenuating FMD and eNOS expression, and may contribute to low arteriolar density. These findings open important perspectives regarding the mechanism involved in the pathophysiology of genetic diseases related to premature stop codons such as DMD.
Figures
Figure 1
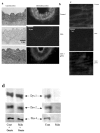
Immunolocalization of dystrophin in carotid arteries (revealed by peroxidase, a) and in mesenteric resistance arteries (Texas-red immunofluorescence, b) was performed using Dys2 anti-dystrophin antibodies. Arteries were isolated from control, mdx mice or mdx mice treated for two weeks with gentamicin. In mesenteric resistance arteries dystrophin (Dys2) was localized to the plasma membrane of endothelial cells, using immunofluorescence and confocal microscopy. This was performed in arteries perfused under a pressure of 75 mmHg and a flow of 50 μl/min (c). (n=6 mice per group). Due to the movement generated by the perfusion of the arteries in physiological conditions, the scanning of the vascular wall was performed at a speed of 10 images/sec. The expression of dystrophin was determined in carotid arteries, using western-blot analysis. Dys1, Dys2 and Dys3 antibodies were directed against C-terminus, N-terminus and mid-rod domains of dystrophin, respectively (d and e). (n=8 mice per group). *P <0.001; two-factor ANOVA, versus control. #P <0.001; two-factor ANOVA, non-treated versus gentamicin-treated mdx mice.
Figure 1
Immunolocalization of dystrophin in carotid arteries (revealed by peroxidase, a) and in mesenteric resistance arteries (Texas-red immunofluorescence, b) was performed using Dys2 anti-dystrophin antibodies. Arteries were isolated from control, mdx mice or mdx mice treated for two weeks with gentamicin. In mesenteric resistance arteries dystrophin (Dys2) was localized to the plasma membrane of endothelial cells, using immunofluorescence and confocal microscopy. This was performed in arteries perfused under a pressure of 75 mmHg and a flow of 50 μl/min (c). (n=6 mice per group). Due to the movement generated by the perfusion of the arteries in physiological conditions, the scanning of the vascular wall was performed at a speed of 10 images/sec. The expression of dystrophin was determined in carotid arteries, using western-blot analysis. Dys1, Dys2 and Dys3 antibodies were directed against C-terminus, N-terminus and mid-rod domains of dystrophin, respectively (d and e). (n=8 mice per group). *P <0.001; two-factor ANOVA, versus control. #P <0.001; two-factor ANOVA, non-treated versus gentamicin-treated mdx mice.
Figure 2
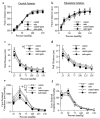
Passive vascular responses to pressure in carotid (left panel) and mesenteric resistance arteries (right panel) isolated from control or mdx mice treated for two weeks with gentamicin (genta, 34 mg/kg/day/14days). Arteries were submitted to stepwise increases in pressure when bathed in a Ca2+-free physiological salt solution containing EGTA (2 mmol/L) and sodium nitroprusside (10 μM), thus defining the passive arterial diameter, expressed as active tone (passive diameter - active diameter 6,14 (a, b). Arterial wall thickness was determined the same arteries (c, d). Cross-sectional compliance (e and f) was calculated from the diameter values shown in a and b. n=10 per group. *P <0.05; two-factor ANOVA.
Figure 3
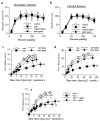
Myogenic tone determined in carotid (a) and mesenteric resistance arteries (b) isolated from control or mdx mice treated for two weeks with gentamicin (genta, n=10 per group). Responses to flow were then determined in mesenteric (c) and carotid arteries (d) under a pressure of 75 mmHg. In another group of experiments, the same experiments (flow-mediated dilation) were performed in vimentin-null mice mesenteric arteries (e). *P <0.05; two-factor ANOVA, mdx versus control (c and d) or vim+/+ versus vim−/− (e)
Figure 4
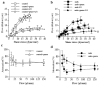
Effect of NO-synthesis blockade with L-NAME (LN) on flow-mediated dilation in mesenteric resistance arteries isolated from control (a) or mdx mice (b) treated for two weeks with gentamicin (genta). Data are represented as μm dilation (a and b) and as percentage inhibition of flow-mediated dilation by L-NAME (in control, c, and mdx, d, mice) (n=10 per group). NO-synthase expression, determined in carotid arteries is shown in e. #P <0.001; two-factor ANOVA, non-treated versus gentamicin-treated mice. *P <0.01; two-factor ANOVA, versus control.
Similar articles
- Defect in microvascular adaptation to chronic changes in blood flow in mice lacking the gene encoding for dystrophin.
Loufrani L, Levy BI, Henrion D. Loufrani L, et al. Circ Res. 2002 Dec 13;91(12):1183-9. doi: 10.1161/01.res.0000047505.11002.81. Circ Res. 2002. PMID: 12480820 - Flow (shear stress)-induced endothelium-dependent dilation is altered in mice lacking the gene encoding for dystrophin.
Loufrani L, Matrougui K, Gorny D, Duriez M, Blanc I, Lévy BI, Henrion D. Loufrani L, et al. Circulation. 2001 Feb 13;103(6):864-70. doi: 10.1161/01.cir.103.6.864. Circulation. 2001. PMID: 11171796 Free PMC article. - Key role of alpha(1)beta(1)-integrin in the activation of PI3-kinase-Akt by flow (shear stress) in resistance arteries.
Loufrani L, Retailleau K, Bocquet A, Dumont O, Danker K, Louis H, Lacolley P, Henrion D. Loufrani L, et al. Am J Physiol Heart Circ Physiol. 2008 Apr;294(4):H1906-13. doi: 10.1152/ajpheart.00966.2006. Epub 2008 Feb 1. Am J Physiol Heart Circ Physiol. 2008. PMID: 18245559
Cited by
- Angiopoietin 1 Attenuates Dysregulated Angiogenesis in the Gastrocnemius of DMD Mice.
McClennan A, Hoffman L. McClennan A, et al. Int J Mol Sci. 2024 Nov 4;25(21):11824. doi: 10.3390/ijms252111824. Int J Mol Sci. 2024. PMID: 39519374 Free PMC article. - Cellular interactions and microenvironment dynamics in skeletal muscle regeneration and disease.
Rodríguez C, Timóteo-Ferreira F, Minchiotti G, Brunelli S, Guardiola O. Rodríguez C, et al. Front Cell Dev Biol. 2024 May 22;12:1385399. doi: 10.3389/fcell.2024.1385399. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38840849 Free PMC article. Review. - NTPDase1/CD39 Ectonucleotidase Is Necessary for Normal Arterial Diameter Adaptation to Flow.
Favre J, Roy C, Guihot AL, Drouin A, Laprise M, Gillis MA, Robson SC, Thorin E, Sévigny J, Henrion D, Kauffenstein G. Favre J, et al. Int J Mol Sci. 2023 Oct 10;24(20):15038. doi: 10.3390/ijms242015038. Int J Mol Sci. 2023. PMID: 37894719 Free PMC article. - Editorial: Molecular physiology of smooth muscle cells.
Schneider H. Schneider H. Front Physiol. 2023 May 30;14:1223278. doi: 10.3389/fphys.2023.1223278. eCollection 2023. Front Physiol. 2023. PMID: 37324402 Free PMC article. No abstract available.
References
- Bevan JA, Laher I. Pressure and flow-dependent vascular tone. Faseb J. 1991;9:2267. - PubMed
- Welling WL, Zupka MY, Welling DJ. Mechanical properties of basement membrane. News Physiol Sci. 1995;10:30.
- Oike M, Schwarz G, Sehrer J, Jost M, Gerke V, Weber K, Droogmans G, Nilius B. Cytoskeletal modulation of the response to mechanical stimulation in human vascular endothelial cells. Pflugers Arch. 1994;428(5–6):569. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources