Identification of an N-terminal amino acid of the CLC-3 chloride channel critical in phosphorylation-dependent activation of a CaMKII-activated chloride current - PubMed (original) (raw)
Identification of an N-terminal amino acid of the CLC-3 chloride channel critical in phosphorylation-dependent activation of a CaMKII-activated chloride current
N C Robinson et al. J Physiol. 2004.
Abstract
CLC-3, a member of the CLC family of chloride channels, mediates function in many cell types in the body. The multifunctional calcium-calmodulin-dependent protein kinase II (CaMKII) has been shown to activate recombinant CLC-3 stably expressed in tsA cells, a human embryonic kidney cell line derivative, and natively expressed channel protein in a human colonic tumour cell line T84. We examined the CaMKII-dependent regulation of CLC-3 in a smooth muscle cell model as well as in the human colonic tumour cell line, HT29, using whole-cell voltage clamp. In CLC-3-expressing cells, we observed the activation of a Cl(-) conductance following intracellular introduction of the isolated autonomous CaMKII into the voltage-clamped cell via the patch pipette. The CaMKII-dependent Cl(-) conductance was not observed following exposure of the cells to 1 microm autocamtide inhibitory peptide (AIP), a selective inhibitor of CaMKII. Arterial smooth muscle cells express a robust CaMKII-activated Cl(-) conductance; however, CLC-3(-/-) cells did not. The N-terminus of CLC-3, which contains a CaMKII consensus sequence, was phosphorylated by CaMKII in vitro, and mutation of the serine at position 109 (S109A) abolished the CaMKII-dependent Cl(-) conductance, indicating that this residue is important in the gating of CLC-3 at the plasma membrane.
Figures
Figure 1. CaMKII-activated Cl− conductance is absent in CLC-3−/− cells
A, representative currents in CLC-3−/−versus CLC-3+/+ mouse aorta smooth muscle cells were recorded at a minimum (Basal) and peak steady-state current level in the presence of CaMKII and 1 μ
m
carbachol ± 1 μ
m
Myr-AIP. Autonomously active CaMKII (10 μg ml−1) was introduced into the cell via the patch pipette. Carbachol was perfused onto the cells following basal recording. Cells treated with Myr-AIP were incubated prior to recording for 10 min. B, current–voltage relationship for currents recorded in A. C, summary of CaMKII-mediated activation in CLC-3−/−versus CLC-3+/+ mouse aorta smooth muscle cells. D, summary of carbachol-mediated activation in CLC-3−/−versus CLC-3+/+ mouse aorta smooth muscle cells. Data are expressed as mean ±
s.e.m.
, with number of cells examined given in parentheses above each bar. E, protein obtained from homogenates of aortae or cultured aortic VSM cells used to identify native CLC-3 protein. Immunoblots confirm the depletion of CLC-3 protein in CLC-3−/− lanes (CLC-3, 90 kDa).
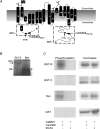
Figure 2. The N-terminus, but not the C-terminus, of CLC-3 is phosphorylated in vitro by CaMKII
A, membrane topology of CLC-3 indicating the location of the Flag epitope, GST fusion constructs, and antibody recognition sites. Membrane topology was adapted from crystal structures of two bacterial CLC channels, StCLC and EcCLC (Dutzler et al. 2002). hCLC-3, StCLC, and EcCLC sequences were aligned using ClustalW (European Bioinformatics Institute) to determine regions of similarity. GST fusion proteins were constructed with the N- (1M to 122L) or C- (661R to 818N) terminal regions of human long form CLC-3. B, in vitro phosphorylation of CLC-3 by CaMKII. The cell lysate of tsA cells stably transfected with hCLC-3 was immunoprecipitated with α-hCLC-3730–744. The precipitated CLC-3 or rabbit synapsin was phosphorylated with CaMKII in the presence of [γ-32P]ATP, and resolved on SDS-PAGE. The resulting gel bands were detected using autoradiography (arrows: CLC-3, 120 kDa (glycosylated), synapsin (Syn) ∼84 kDa. C, in vitro phosphorylation of GST CLC-3 fusion proteins by CaMKII. The GST CLC-3 or rabbit synapsin was phosphorylated with CaMKII in the presence of [γ-32P]ATP, and resolved on SDS-PAGE. The resulting gel bands were detected using autoradiography (phosphorylation) and Coomassie blue to detect the presence of protein. Similar results were obtained in three experiments.
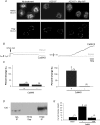
Figure 3. Trafficking of CLC-3 is not CaMKII dependent and is not necessary for kinase-dependent conductance increase
A: upper panels, HT29 cells expressing native CLC-3 were permeabilized and localization was visualized with α-hCLC-3730–744. PBS-treated cells (Control) show a perinuclear distribution of CLC-3. Cells treated with 10 μ
m
A23187, a Ca2+ ionophore, showed a diffuse cytosolic distribution of CLC-3. Cells treated with 1 μ
m
of the specific CaMKII inhibitor, autocamtide-2 inhibitory peptide (AIP), prior to treatment with A23187, showed the same diffuse distribution as those treated with A23187 alone; lower panels, HT29 cells were transfected with Flag-CLC-3, and in these non-permeabilized cells there is no apparent difference in fluorescence labelling following intracellular Ca2+ elevation (same treatment conditions as in non-transfected cells). Similar results were seen in three experiments.B, representative traces of membrane capacitance (_C_m) and membrane conductance (_G_m) with (CaMKII) or without (Basal) autonomous CaMKII in the pipette. The holding potential was −5 mV. The arrow indicates initiation of whole-cell configuration. C, summary of changes in capacitance and conductance in the presence and absence Trafficking of CLC-3 is not CaMKII dependent and is not necessary for kinase-dependent conductance increase of CaMKII. The percentage change is relative to baseline levels following initiation of whole-cell configuration. D, immunoblot of CLC-3 immunoprecipitated (IP) with α-hCLC-3730–744 from HT29 cells or CLC-3 stably transfected tsA cells, blotted with α-hCLC-359–74 (molecular mass, ∼120 kDa, glycosylated). No CLC-3 protein was detected in HT29 supernatant (Sup) lane. E, summary of CaMKII-activated Cl− current densities in HT29 cells, with (CaMKII ± AIP) or without (Basal) autonomous CaMKII in the pipette. AIP (1 μ
m)
was included in the pipette solution where indicated. *Significant difference (P < 0.01). Data are expressed as mean ±
s.e.m.,
with number of cells examined given in parentheses above each bar.
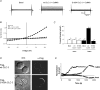
Figure 4. Mutation at amino acid S109 in CLC-3 abolishes CaMKII activation of _I_Cl, but does not inhibit trafficking to the membrane
A, representative currents in transfected wt CLC-3 versus S109A CLC-3 tsA cells were recorded at a minimum (Basal) and after they reached steady state in the presence of CaMKII. Autonomously active CaMKII was introduced into the cell via the patch pipette. CLC-3 (wt or S109A) was transfected into tsA cells 48 h prior to experiment. Green fluorescent protein (GFP) was cotransfected to identify positive cells. B, current–voltage relationship for A. C, summary of CaMKII-mediated activation in tsA cells transiently transfected with wt CLC-3 versus S109A CLC-3 or mock-transfected cells. Data are expressed as mean ±
s.e.m.
, with number of cells examined given in parentheses above each bar. D, immunostaining of non-permeabilized tsA cells transfected with wt or S109A Flag-CLC-3. Plasma membrane localization is visualized after incubation with an anti-Flag antibody. E, representative time course of activation in three CLC-3 stably transfected tsA cells with (CaMKII) and without (Basal) CaMKII in the pipette, and 1 μ
m
carbachol in the bath. Time zero for the CaMKII trace is initiation of whole-cell configuration; time zero for carbachol trace is initiation of carbachol superfusion into the bath.
Similar articles
- Regulation of human CLC-3 channels by multifunctional Ca2+/calmodulin-dependent protein kinase.
Huang P, Liu J, Di A, Robinson NC, Musch MW, Kaetzel MA, Nelson DJ. Huang P, et al. J Biol Chem. 2001 Jun 8;276(23):20093-100. doi: 10.1074/jbc.M009376200. Epub 2001 Mar 26. J Biol Chem. 2001. PMID: 11274166 - Differential regulation of Ca(2+)-activated Cl(-) currents in rabbit arterial and portal vein smooth muscle cells by Ca(2+)-calmodulin-dependent kinase.
Greenwood IA, Ledoux J, Leblanc N. Greenwood IA, et al. J Physiol. 2001 Jul 15;534(Pt. 2):395-408. doi: 10.1111/j.1469-7793.2001.00395.x. J Physiol. 2001. PMID: 11454959 Free PMC article. - Protein kinase A activation phosphorylates the rat ClC-2 Cl- channel but does not change activity.
Park K, Begenisich T, Melvin JE. Park K, et al. J Membr Biol. 2001 Jul 1;182(1):31-7. doi: 10.1007/s00232-001-0026-0. J Membr Biol. 2001. PMID: 11426297 - Coupling gating with ion permeation in ClC channels.
Chen TY. Chen TY. Sci STKE. 2003 Jun 24;2003(188):pe23. doi: 10.1126/stke.2003.188.pe23. Sci STKE. 2003. PMID: 12824475 Review. - Structural basis for ion conduction and gating in ClC chloride channels.
Dutzler R. Dutzler R. FEBS Lett. 2004 Apr 30;564(3):229-33. doi: 10.1016/S0014-5793(04)00210-8. FEBS Lett. 2004. PMID: 15111101 Review.
Cited by
- Ca(2+)-activated chloride channels go molecular.
Pusch M. Pusch M. J Gen Physiol. 2004 Apr;123(4):323-5. doi: 10.1085/jgp.200409053. J Gen Physiol. 2004. PMID: 15051804 Free PMC article. Review. No abstract available. - Identification and functional characterization of a voltage-gated chloride channel and its novel splice variant in taste bud cells.
Huang L, Cao J, Wang H, Vo LA, Brand JG. Huang L, et al. J Biol Chem. 2005 Oct 28;280(43):36150-7. doi: 10.1074/jbc.M507706200. Epub 2005 Aug 29. J Biol Chem. 2005. PMID: 16129671 Free PMC article. - The kinetics of synaptic vesicle reacidification at hippocampal nerve terminals.
Atluri PP, Ryan TA. Atluri PP, et al. J Neurosci. 2006 Feb 22;26(8):2313-20. doi: 10.1523/JNEUROSCI.4425-05.2006. J Neurosci. 2006. PMID: 16495458 Free PMC article. - CaMKII inhibition hyperpolarizes membrane and blocks nitrergic IJP by closing a Cl(-) conductance in intestinal smooth muscle.
He XD, Goyal RK. He XD, et al. Am J Physiol Gastrointest Liver Physiol. 2012 Jul 15;303(2):G240-6. doi: 10.1152/ajpgi.00102.2012. Epub 2012 Apr 26. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22538403 Free PMC article. - CLC-3 chloride channels moderate long-term potentiation at Schaffer collateral-CA1 synapses.
Farmer LM, Le BN, Nelson DJ. Farmer LM, et al. J Physiol. 2013 Feb 15;591(4):1001-15. doi: 10.1113/jphysiol.2012.243485. Epub 2012 Nov 19. J Physiol. 2013. PMID: 23165767 Free PMC article.
References
- Arreola J, Melvin J, Begenisich T. Differences in regulation of Ca2+−activated Cl− channels in colonic and parotid secretory cells. Am J Physiol. 1998;274:C161–C166. - PubMed
- Barg S, Huang P, Eliasson L, Nelson DJ, Obermuller S, Rorsman P, et al. Priming of insulin granules for exocytosis by granular Cl− uptake and acidification. J Cell Sci. 2001;114:2145–2154. - PubMed
- Breit S, Kolb H, Apfel H. Regulation of ion channels in rat hepatocytes. Pflugers Arch. 1998;435:203–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK057882/DK/NIDDK NIH HHS/United States
- R01 HL062483/HL/NHLBI NIH HHS/United States
- R01 DK57882/DK/NIDDK NIH HHS/United States
- R01 HL62483/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous