Kinetics of Mg2+ unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity - PubMed (original) (raw)
Kinetics of Mg2+ unblock of NMDA receptors: implications for spike-timing dependent synaptic plasticity
Björn M Kampa et al. J Physiol. 2004.
Abstract
The time course of Mg(2+) block and unblock of NMDA receptors (NMDARs) determines the extent they are activated by depolarization. Here, we directly measure the rate of NMDAR channel opening in response to depolarizations at different times after brief (1 ms) and sustained (4.6 s) applications of glutamate to nucleated patches from neocortical pyramidal neurons. The kinetics of Mg(2+) unblock were found to be non-instantaneous and complex, consisting of a prominent fast component (time constant approximately 100 micros) and slower components (time constants 4 and approximately 300 ms), the relative amplitudes of which depended on the timing of the depolarizing pulse. Fitting a kinetic model to these data indicated that Mg(2+) not only blocks the NMDAR channel, but reduces both the open probability and affinity for glutamate, while enhancing desensitization. These effects slow the rate of NMDAR channel opening in response to depolarization in a time-dependent manner such that the slower components of Mg(2+) unblock are enhanced during depolarizations at later times after glutamate application. One physiological consequence of this is that brief depolarizations occurring earlier in time after glutamate application are better able to open NMDAR channels. This finding has important implications for spike-timing-dependent synaptic plasticity (STDP), where the precise (millisecond) timing of action potentials relative to synaptic inputs determines the magnitude and sign of changes in synaptic strength. Indeed, we find that STDP timing curves of NMDAR channel activation elicited by realistic dendritic action potential waveforms are narrower than expected assuming instantaneous Mg(2+) unblock, indicating that slow Mg(2+) unblock of NMDAR channels makes the STDP timing window more precise.
Figures
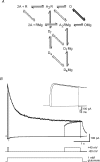
Figure 1. The magnitude of slow unblock depends on the timing of depolarization
A, superimposed NMDAR currents during 1 ms applications of 1 m
m
glutamate in the presence of 1 m
m
Mg2+ at holding potentials of −60 and +40 mV (black), and during a step from −60 to +40 mV starting 10 ms after glutamate application (grey). The timing of glutamate application and the voltage step from −60 to +40 mV are indicated at the bottom. B, same as in A but during a long (4.6 s) application of glutamate. C, same as A and B during a 1 s voltage step from −60 to +40 mV starting 3.5 s after a long (4.6 s) application of 1 m
m
glutamate (grey). Inset shows same voltage step applied to a different patch in solution without external Mg2+. D, NMDAR current at +40 mV divided by that during steps from −60 to +40 mV starting 10 ms (‘early step’; data from B) or 3.5 s (‘late step’; data from C) after long (4.6 s) applications of 1 m
m
glutamate. Black traces show multiexponential fits. E, pooled data (n = 5) of the time constant (columns) and relative amplitude (•) of multiexponential fits to Mg2+ unblock during steps from −60 to +40 mV starting 10 ms (‘early step’; dark grey) and 3.5 s (‘late step’; light grey) after long applications of glutamate. Steps at 10 ms were fitted with a double exponential, whereas a triple exponential was required to fit steps at 3.5 s.
Figure 2. Markov model of NMDAR activation
A, reaction scheme shows two molecules of glutamate (A) binding to the NMDA receptor (R), opening of the channel (O), and transition to fast and slow desensitization states (Df and Ds). Mg2+ binds to all five states, although binding is much more rapid to the open state (thick lines) than to the other states (dashed lines). B, recorded and simulated NMDAR current during 4.6 s applications of 1 m
m
glutamate in the presence of 1 m
m
Mg2+ at holding potentials of −60 and +40 mV (black), and during a step from −60 to +40 mV starting 10 ms (see inset with expanded time scale) or 3.5 s after the glutamate application (grey). Fitted transients (smooth curves) generated by the model shown in A are superimposed on the data.
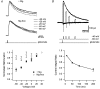
Figure 3. Magnesium affects NMDAR channel activation
A, normalized (at peak), superimposed NMDAR currents during 1 ms applications of 1 m
m
glutamate at the indicated holding potentials in the presence of 1 m
m
Mg2+ (top) and in Mg2+-free solution (middle). Bottom, average duration at half-amplitude of NMDAR currents evoked by 1 ms applications of 1 m
m
glutamate normalized to that at +40 mV and plotted against the holding potential in solutions with (•) and without (○) Mg2+ (n = 6). B, top, superimposed NMDAR currents during 1 ms applications of 1 m
m
glutamate at +40 mV, and during 10 ms voltage steps from −60 to +40 mV occurring 10, 50, 100 or 200 ms after onset of glutamate application. Bottom, ratio of the current integral during 10 ms voltage steps (as in B) relative to the integral (over the same time) of the response obtained at a holding potential of +40 mV (n = 6).
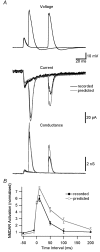
Figure 4. NMDAR activation during STDP induction protocols
A, top, dendritic voltage (650 μm from the soma) during pairing of an EPSP with a backpropagating AP 10 or 50 ms after EPSP onset. Middle, recorded (black) and predicted (grey, assuming instantaneous Mg2+ unblock) NMDAR current during EPSP–AP pairing. Bottom, recorded (black) and predicted (grey) NMDAR conductance. B, NMDAR activation during EPSP–AP pairing at different time intervals for recorded (black) and predicted (grey) NMDAR currents. Currents were normalized in each patch to the response during 1 ms applications of 1 m
m
glutamate at +40 mV and the area around the conductance peak (3 ms before to 10 ms after) was used to quantify NMDAR activation.
Similar articles
- A slow fraction of Mg2+ unblock of NMDA receptors limits their contribution to spike generation in cortical pyramidal neurons.
Vargas-Caballero M, Robinson HP. Vargas-Caballero M, et al. J Neurophysiol. 2003 May;89(5):2778-83. doi: 10.1152/jn.01038.2002. Epub 2003 Jan 22. J Neurophysiol. 2003. PMID: 12611983 - Temperature dependence of N-methyl-D-aspartate receptor channels and N-methyl-D-aspartate receptor excitatory postsynaptic currents.
Korinek M, Sedlacek M, Cais O, Dittert I, Vyklicky L Jr. Korinek M, et al. Neuroscience. 2010 Feb 3;165(3):736-48. doi: 10.1016/j.neuroscience.2009.10.058. Epub 2009 Oct 31. Neuroscience. 2010. PMID: 19883737 - Effects of divalent cations on slow unblock of native NMDA receptors in mouse neocortical pyramidal neurons.
Kim NK, Robinson HP. Kim NK, et al. Eur J Neurosci. 2011 Jul;34(2):199-212. doi: 10.1111/j.1460-9568.2011.07768.x. Epub 2011 Jul 4. Eur J Neurosci. 2011. PMID: 21722211 - The chemical biology of clinically tolerated NMDA receptor antagonists.
Chen HS, Lipton SA. Chen HS, et al. J Neurochem. 2006 Jun;97(6):1611-26. doi: 10.1111/j.1471-4159.2006.03991.x. J Neurochem. 2006. PMID: 16805772 Review.
Cited by
- Hybrid stochastic simulations of intracellular reaction-diffusion systems.
Kalantzis G. Kalantzis G. Comput Biol Chem. 2009 Jun;33(3):205-15. doi: 10.1016/j.compbiolchem.2009.03.002. Epub 2009 Apr 2. Comput Biol Chem. 2009. PMID: 19414282 Free PMC article. - Targeting NMDA Receptor Complex in Management of Epilepsy.
Sivakumar S, Ghasemi M, Schachter SC. Sivakumar S, et al. Pharmaceuticals (Basel). 2022 Oct 21;15(10):1297. doi: 10.3390/ph15101297. Pharmaceuticals (Basel). 2022. PMID: 36297409 Free PMC article. Review. - The Potential of Corticospinal-Motoneuronal Plasticity for Recovery after Spinal Cord Injury.
Jo HJ, Richardson MSA, Oudega M, Perez MA. Jo HJ, et al. Curr Phys Med Rehabil Rep. 2020 Sep;8(3):293-298. doi: 10.1007/s40141-020-00272-6. Epub 2020 Aug 4. Curr Phys Med Rehabil Rep. 2020. PMID: 33777502 Free PMC article. - Quantitative dynamics and spatial profile of perisomatic GABAergic input during epileptiform synchronization in the CA1 hippocampus.
Marchionni I, Maccaferri G. Marchionni I, et al. J Physiol. 2009 Dec 1;587(Pt 23):5691-708. doi: 10.1113/jphysiol.2009.179945. Epub 2009 Oct 19. J Physiol. 2009. PMID: 19840998 Free PMC article. - NMDA receptors: linking physiological output to biophysical operation.
Iacobucci GJ, Popescu GK. Iacobucci GJ, et al. Nat Rev Neurosci. 2017 Mar 17;18(4):236-249. doi: 10.1038/nrn.2017.24. Nat Rev Neurosci. 2017. PMID: 28303017 Free PMC article. Review.
References
- Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. - PubMed
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
- Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases