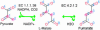The metabolic world of Escherichia coli is not small - PubMed (original) (raw)
The metabolic world of Escherichia coli is not small
Masanori Arita. Proc Natl Acad Sci U S A. 2004.
Abstract
To elucidate the organizational and evolutionary principles of the metabolism of living organisms, recent studies have addressed the graph-theoretic analysis of large biochemical networks responsible for the synthesis and degradation of cellular building blocks [Jeong, H., Tombor, B., Albert, R., Oltvai, Z. N. & Barabási, A. L. (2000) Nature 407, 651-654; Wagner, A. & Fell, D. A. (2001) Proc. R. Soc. London Ser. B 268, 1803-1810; and Ma, H.-W. & Zeng, A.-P. (2003) Bioinformatics 19, 270-277]. In such studies, the global properties of the network are computed by considering enzymatic reactions as links between metabolites. However, the pathways computed in this manner do not conserve their structural moieties and therefore do not correspond to biochemical pathways on the traditional metabolic map. In this work, we reassessed earlier results by digitizing carbon atomic traces in metabolic reactions annotated for Escherichia coli. Our analysis revealed that the average path length of its metabolism is much longer than previously thought and that the metabolic world of this organism is not small in terms of biosynthesis and degradation.
Figures
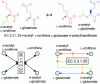
Fig. 1.
Two representations of the EC 2.3.1.35 reaction. In this reaction, the acetyl moiety of _N_-acetyl
l
-ornithine is transferred to
l
-glutamate to form _N_-acetyl
l
-glutamate. (Lower Left) In the scheme of Jeong et al. (7), its two substrates and two products are equally linked to the object representing the EC number, irrespective of their structural changes. (Lower Right) In our scheme, conserved substructural moieties, coded by color, are computationally detected, and each link is associated with the information of which atom goes where.
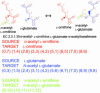
Fig. 2.
Three atomic mappings for the EC 2.3.1.35 reaction. Each reaction formula is decomposed to a set of substructural correspondences coded by color: each color indicates a set of atomic position pairs called an atomic mapping. Atomic positions are line numbers in the MOL file format files (see Methods for details) and are not generalizable to other metabolic databases.
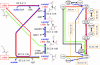
Fig. 3.
Graph representation of the ornithine biosynthetic pathway. In ornithine biosynthesis,
l
-ornithine (
l
-Orn) is synthesized from
l
-glutamate (
l
-Glu) through five reactions. Red arrows indicate the transfer of the carbon skeleton of
l
-glutamate, blue arrows indicate the transfer of acetyl moiety, and green arrows indicate the transfer of a nitrogen atom from
l
-glutamate. (Left) In the traditional metabolic map, multiple substrates and products are involved in each reaction, and their structural relationships are implicit. (Right) In our graph representation, physically related metabolites are linked with atomic mappings (red and blue arrows), and each reaction corresponds to a set of mappings (orange links). Note that the mapping between
l
-glutamate and _N_-acetyl
l
-glutamate (_N_-Ace
l
-Glu) is shared by two reactions (EC 2.3.1.1 and EC 2.3.1.35). The mapping between
l
-ornithine and _N_-acetyl
l
-ornithine (_N_-Ace
l
-Orn) is also shared. p, Phosphate; sa, semialdehyde.
Fig. 4.
Pathway from pyruvate to fumarate. Highlighted positions show the traces of two carbon atoms in pyruvate (positions 0 and 1). Because the two positions in fumarate ( and 3) are equivalent, all highlighted positions become equivalent when reactions are considered reversible.
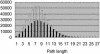
Fig. 5.
Distribution of pathway length. Filled bars indicate the population of pathways when the direction of reactions is considered (i.e., directed graph). Open bars indicate the population when all reactions are considered reversible (undirected graph).
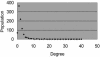
Fig. 6.
Degree distribution of the graph. Degree corresponds to the number of structural changes, not frequencies.
Similar articles
- Inferring meaningful pathways in weighted metabolic networks.
Croes D, Couche F, Wodak SJ, van Helden J. Croes D, et al. J Mol Biol. 2006 Feb 10;356(1):222-36. doi: 10.1016/j.jmb.2005.09.079. Epub 2005 Oct 17. J Mol Biol. 2006. PMID: 16337962 - Protein complexes and functional modules in molecular networks.
Spirin V, Mirny LA. Spirin V, et al. Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12123-8. doi: 10.1073/pnas.2032324100. Epub 2003 Sep 29. Proc Natl Acad Sci U S A. 2003. PMID: 14517352 Free PMC article. - An extended bioreaction database that significantly improves reconstruction and analysis of genome-scale metabolic networks.
Stelzer M, Sun J, Kamphans T, Fekete SP, Zeng AP. Stelzer M, et al. Integr Biol (Camb). 2011 Nov;3(11):1071-86. doi: 10.1039/c1ib00008j. Epub 2011 Sep 27. Integr Biol (Camb). 2011. PMID: 21952610 - Metabolic systems cost-benefit analysis for interpreting network structure and regulation.
Carlson RP. Carlson RP. Bioinformatics. 2007 May 15;23(10):1258-64. doi: 10.1093/bioinformatics/btm082. Epub 2007 Mar 7. Bioinformatics. 2007. PMID: 17344237 - Pathway identification by network pruning in the metabolic network of Escherichia coli.
Gerlee P, Lizana L, Sneppen K. Gerlee P, et al. Bioinformatics. 2009 Dec 15;25(24):3282-8. doi: 10.1093/bioinformatics/btp575. Epub 2009 Oct 6. Bioinformatics. 2009. PMID: 19808881
Cited by
- A strategy to detect metabolic changes induced by exposure to chemicals from large sets of condition-specific metabolic models computed with enumeration techniques.
Fresnais L, Perin O, Riu A, Grall R, Ott A, Fromenty B, Gallardo JC, Stingl M, Frainay C, Jourdan F, Poupin N. Fresnais L, et al. BMC Bioinformatics. 2024 Jul 11;25(1):234. doi: 10.1186/s12859-024-05845-z. BMC Bioinformatics. 2024. PMID: 38992584 Free PMC article. - Genetic basis and selection of glyceollin elicitation in wild soybean.
Yasmin F, Zhang H, Leamy L, Wang B, Winnike J, Reid RW, Brouwer CR, Song BH. Yasmin F, et al. Front Plant Sci. 2024 Feb 28;15:1240981. doi: 10.3389/fpls.2024.1240981. eCollection 2024. Front Plant Sci. 2024. PMID: 38481402 Free PMC article. - The EcoCyc Database (2023).
Karp PD, Paley S, Caspi R, Kothari A, Krummenacker M, Midford PE, Moore LR, Subhraveti P, Gama-Castro S, Tierrafria VH, Lara P, Muñiz-Rascado L, Bonavides-Martinez C, Santos-Zavaleta A, Mackie A, Sun G, Ahn-Horst TA, Choi H, Covert MW, Collado-Vides J, Paulsen I. Karp PD, et al. EcoSal Plus. 2023 Dec 12;11(1):eesp00022023. doi: 10.1128/ecosalplus.esp-0002-2023. Epub 2023 May 11. EcoSal Plus. 2023. PMID: 37220074 Free PMC article. Review. - BioSIMP: Using Software Testing Techniques for Sampling and Inference in Biological Organisms.
Cashman M, Catlett JL, Cohen MB, Buan NR, Sakkaff Z, Pierobon M, Kelley CA. Cashman M, et al. SE4Science 2017 (2017). 2017 May;2017:2-8. doi: 10.1109/se4science.2017.9. Epub 2017 Jul 3. SE4Science 2017 (2017). 2017. PMID: 36848304 Free PMC article. - Metabolic Pathway Analysis: Advantages and Pitfalls for the Functional Interpretation of Metabolomics and Lipidomics Data.
Tsouka S, Masoodi M. Tsouka S, et al. Biomolecules. 2023 Jan 27;13(2):244. doi: 10.3390/biom13020244. Biomolecules. 2023. PMID: 36830612 Free PMC article.
References
- Watts, D. J. & Strogatz, S. H. (1998) Nature 393, 440-442. - PubMed
- Strogatz, S. H. (2001) Nature 410, 268-276. - PubMed
- Fell, D. A. & Wagner, A. (2000) Nat. Biotechnol. 18, 1121-1122. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources