A new gamma-interferon-inducible promoter and splice variants of an anti-angiogenic human tRNA synthetase - PubMed (original) (raw)
A new gamma-interferon-inducible promoter and splice variants of an anti-angiogenic human tRNA synthetase
Jianming Liu et al. Nucleic Acids Res. 2004.
Abstract
Two forms of human tryptophanyl-tRNA synthetase (TrpRS) are produced in vivo through alternative mRNA splicing. The two forms, full-length TrpRS and mini TrpRS, are catalytically active, but are distinguished by the striking anti-proliferative and anti-angiogenic activity specific to mini TrpRS. Here we describe two new splice variants of human TrpRS mRNA. Their production was strongly regulated by gamma-interferon (IFN-gamma), an anti-proliferative cytokine known to stimulate the expression of other anti-angiogenic factors. A new IFN-gamma-sensitive promoter was demonstrated to drive production of these splice variants. In human endothelial cells, both the newly discovered and a previously reported promoter were shown to respond specifically to IFN-gamma and not to other cytokines such as tumor necrosis factor-alpha, transforming growth factor-beta, interleukin-4 or erythropoietin. In addition, both promoters were stimulated by the 'downstream' interferon regulatory factor 1 that, in turn, is known to be regulated by the 'upstream' signal transducer and activator of transcription 1alpha subunit. Thus, the tandem promoters provide a dual system to regulate expression and alternative splicing of human TrpRS in vivo.
Figures
Figure 1
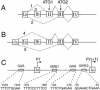
Schematic presentation of genomic DNA organization and alternative splicing of the human TrpRS gene. Boxes and lines represent exons and introns, respectively. Discontinuous lines indicate major spliced forms of TrpRS mRNA. Translation initiated from ATG1 produces full-length TrpRS protein, the translation product from ATG2 corresponds to mini TrpRS protein. (A) Three alternative splicing forms of mRNA containing exon Ia (23). (B) Two newly discovered alternative splicing forms of mRNA containing exon –Ia. (C) Schematic presentation of the 5′ regulatory region of human TrpRS. The major transcription start site within exon Ia is referred to as +1. The potential IFN-related regulatory elements and their nucleotide sequences and positions are indicated, several of which were previously described (23,36). The position of exon –Ia is –1458 to –912. Two promoters are indicated as P1 and P2. GAS, γ-activated sequence; ISRE, interferon-stimulated response element.
Figure 2
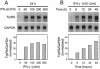
IFN-γ up-regulates TrpRS in HUVECs. (A) Concentration- and (B) time-dependent up-regulation of TrpRS mRNA in HUVECs stimulated with IFN-γ. Total RNA was isolated from the cells stimulated with various amount of IFN-γ for different lengths of time and the levels of mRNAs for TrpRS and GAPDH were analyzed by northern blot hybridization. TrpRS mRNA levels were normalized to GAPDH mRNA levels to control for variations in loading between samples.
Figure 3
RT–PCR analyses of IFN-γ up-regulated TrpRS isoforms in HUVECs. Cells were treated with or without IFN-γ (100 U/ml) for 24 h. Total RNAs were isolated and converted to cDNA. PCR was performed with cDNA (prepared as described in Materials and Methods and diluted 1:100) and analyzed by 1.2% agarose gel electrophoresis. Under these conditions, the differences between the levels of TrpRS mRNA isoforms could be seen. GAPDH is an internal control.
Figure 4
Northern blot analyses of IFN-γ up-regulated TrpRS isoforms expressed in HUVECs. Total RNA was isolated from the cells stimulated with various amounts of IFN-γ for different lengths of time and the levels of mRNAs for TrpRS were analyzed by northern blot hybridization. GAPDH is an internal control for even loading. Specific probes for different mRNA isoforms are illustrated to the left in the figure.
Figure 5
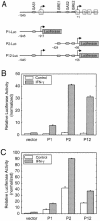
Transient transfection assays. (A) Schematic diagram showing different luciferase reporter constructs. Open boxes represent exons. The DNA sequence of exon –Ia is included in the P12-Luc construct. The putative IFN-related regulatory elements are indicated. GAS, γ-activated sequence; ISRE, interferon-stimulated response element. (B) HeLa cells transfected with TrpRS promoter constructs and incubated with or without IFN-γ (100 U/ml) for 24 h. (C) HUVECs transfected with TrpRS promoter constructs and incubated with or without IFN-γ (100 U/ml) for 24 h. Data shown here are representative of three independent measurements. Results are expressed as fold induction relative to the activity of the empty reporter vector control and represent the mean ± SE (n = 3).
Figure 6
Overexpression of IRF-1 activated TrpRS promoters. HeLa cells were transiently co-transfected with indicated promoter constructs along with IRF-1 or IRF-2 vector for 24 h in the absence or presence of IFN-γ (100 U/ml). (A) P1-Luc; (B) P2-Luc; (C) P12-Luc. Data shown here are representative of three independent measurements. Results are expressed as fold induction relative to the activity of the empty reporter vector control and represent the mean ± SE (n = 3).
Figure 7
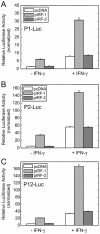
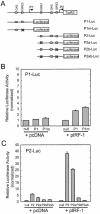
Transient transfection assays. HeLa cells were transiently co-transfected with the indicated promoter constructs along with IRF-1 for 24 h. (A) Schematic diagram showing different luciferase reporter constructs. Open boxes represent exons. The putative IFN-related regulatory elements GAS and ISRE are indicated; (B) P1-Luc; (C) P2-Luc. Data shown here are representative of three independent measurements. Results are expressed as fold induction relative to the activity of the empty reporter vector control and represent the mean ± SE (n = 3).
Similar articles
- Modulation of cellular tryptophan metabolism in human fibroblasts by transforming growth factor-beta: selective inhibition of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA synthetase gene expression.
Yuan W, Collado-Hidalgo A, Yufit T, Taylor M, Varga J. Yuan W, et al. J Cell Physiol. 1998 Oct;177(1):174-86. doi: 10.1002/(SICI)1097-4652(199810)177:1<174::AID-JCP18>3.0.CO;2-D. J Cell Physiol. 1998. PMID: 9731757 - Expression of the rodent-specific alternative splice variant of tryptophanyl-tRNA synthetase in murine tissues and cells.
Miyanokoshi M, Tanaka T, Tamai M, Tagawa Y, Wakasugi K. Miyanokoshi M, et al. Sci Rep. 2013 Dec 11;3:3477. doi: 10.1038/srep03477. Sci Rep. 2013. PMID: 24327169 Free PMC article. - Hypoxia signature of splice forms of tryptophanyl-tRNA synthetase marks pancreatic cancer cells with distinct metastatic abilities.
Paley EL, Paley DE, Merkulova-Rainon T, Subbarayan PR. Paley EL, et al. Pancreas. 2011 Oct;40(7):1043-56. doi: 10.1097/MPA.0b013e318222e635. Pancreas. 2011. PMID: 21926542 - The IFN-γ/miniTrpRS signaling axis: An insight into the pathophysiology of osteoporosis and therapeutic potential.
Biros E, Malabu UH, Vangaveti VN, Birosova E, Moran CS. Biros E, et al. Cytokine Growth Factor Rev. 2022 Apr;64:7-11. doi: 10.1016/j.cytogfr.2022.01.005. Epub 2022 Jan 21. Cytokine Growth Factor Rev. 2022. PMID: 35115234 Review. - Mammalian tryptophanyl-tRNA synthetases.
Kisselev LL. Kisselev LL. Biochimie. 1993;75(12):1027-39. doi: 10.1016/0300-9084(93)90002-a. Biochimie. 1993. PMID: 7515282 Review.
Cited by
- The high-affinity tryptophan uptake transport system in human cells.
Wakasugi K, Yokosawa T. Wakasugi K, et al. Biochem Soc Trans. 2024 Jun 26;52(3):1149-1158. doi: 10.1042/BST20230742. Biochem Soc Trans. 2024. PMID: 38813870 Free PMC article. Review. - Inflammation drives alternative first exon usage to regulate immune genes including a novel iron-regulated isoform of Aim2.
Robinson EK, Jagannatha P, Covarrubias S, Cattle M, Smaliy V, Safavi R, Shapleigh B, Abu-Shumays R, Jain M, Cloonan SM, Akeson M, Brooks AN, Carpenter S. Robinson EK, et al. Elife. 2021 May 28;10:e69431. doi: 10.7554/eLife.69431. Elife. 2021. PMID: 34047695 Free PMC article. - Tryptophan Depletion Modulates Tryptophanyl-tRNA Synthetase-Mediated High-Affinity Tryptophan Uptake into Human Cells.
Yokosawa T, Sato A, Wakasugi K. Yokosawa T, et al. Genes (Basel). 2020 Nov 27;11(12):1423. doi: 10.3390/genes11121423. Genes (Basel). 2020. PMID: 33261077 Free PMC article. - Role of host tRNAs and aminoacyl-tRNA synthetases in retroviral replication.
Jin D, Musier-Forsyth K. Jin D, et al. J Biol Chem. 2019 Apr 5;294(14):5352-5364. doi: 10.1074/jbc.REV118.002957. Epub 2019 Jan 30. J Biol Chem. 2019. PMID: 30700559 Free PMC article. Review. - Tryptophanyl-tRNA synthetase mediates high-affinity tryptophan uptake into human cells.
Miyanokoshi M, Yokosawa T, Wakasugi K. Miyanokoshi M, et al. J Biol Chem. 2018 Jun 1;293(22):8428-8438. doi: 10.1074/jbc.RA117.001247. Epub 2018 Apr 17. J Biol Chem. 2018. PMID: 29666190 Free PMC article.
References
- Schimmel P. (1987) Aminoacyl tRNA synthetases: general scheme of structure–function relationships in the polypeptides and recognition of transfer RNAs. Annu. Rev. Biochem., 56, 125–158. - PubMed
- Moras D. (1992) Structural and functional relationships between aminoacyl-tRNA synthetases. Trends Biochem. Sci., 17, 159–164. - PubMed
- Webster T., Tsai,H., Kula,M., Mackie,G.A. and Schimmel,P. (1984) Specific sequence homology and three-dimensional structure of an aminoacyl transfer RNA synthetase. Science, 226, 1315–1317. - PubMed
- Ludmerer S.W. and Schimmel,P. (1987) Gene for yeast glutamine tRNA synthetase encodes a large amino-terminal extension and provides a strong confirmation of the signature sequence for a group of the aminoacyl-tRNA synthetases. J. Biol. Chem., 262, 10801–10806. - PubMed
- Eriani G., Delarue,M., Poch,O., Gangloff,J. and Moras,D. (1990) Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature, 347, 203–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases