KCNQ2 is a nodal K+ channel - PubMed (original) (raw)
KCNQ2 is a nodal K+ channel
Jérôme J Devaux et al. J Neurosci. 2004.
Abstract
Mutations in the gene encoding the K+ channel KCNQ2 cause neonatal epilepsy and myokymia, indicating that KCNQ2 regulates the excitability of CNS neurons and motor axons, respectively. We show here that KCNQ2 channels are functional components of axon initial segments and nodes of Ranvier, colocalizing with ankyrin-G and voltage-dependent Na+ channels throughout the CNS and PNS. Retigabine, which opens KCNQ channels, diminishes axonal excitability. Linopirdine, which blocks KCNQ channels, prolongs the repolarization of the action potential in neonatal nerves. The clustering of KCNQ2 at nodes and initial segments lags that of ankyrin-G during development, and both ankyrin-G and KCNQ2 can be coimmunoprecipitated in the brain. KCNQ3 is also a component of some initial segments and nodes in the brain. The diminished activity of mutant KCNQ2 channels accounts for neonatal epilepsy and myokymia; the cellular locus of these effects may be axonal initial segments and nodes.
Figures
Figure 1.
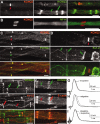
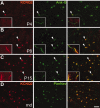
KCNQ2 is localized to PNS nodes of Ranvier. These are images of unfixed teased fibers from adult rat sciatic nerves immunolabeled for KCNQ2 (A–D) or KCNQ3 (A, E, F) and Nav channels α subunits (PanNav) (C), Kv1.2 (D, E), or E-cadherin (F). KCNQ2 and KCNQ3 are distinctly localized (A). A single optical section obtained by confocal microscopy shows that KNCQ2 (red) is localized to the axonal membrane, as shown by comparison to NF-H staining (B, green). KCNQ2 is colocalized with Nav channels at nodes of Ranvier (C), whereas KCNQ3 (A, E, F) is localized at incisures (green double arrows) and outer mesaxons (green arrows), where it is colocalized with E-cadherin (F). Kv1.2 is localized to the axonal membrane at juxtaparanodes (D, green double arrowheads), “juxta-mesaxons” (aligned with the glial inner mesaxon) (E, red arrows), and the “juxta-incisures” (aligned with the inner aspect of incisures) (E, red double arrows), locations that are distinct from those of KCNQ2 and KCNQ3. G_–_I, Compound action potential recorded from 3-month-old rat sciatic nerves. Linopirdine (100 μ
m
) did not change the shape of the CAP recorded from adults (G). By contrast, retigabine (20 μ
m
) delayed the onset of the CAP (H); these effects were blocked by pretreating the nerves with linopirdine (20 μ
m
) (I). Scale bars: B, 5 μm; A, C_–_F, 10 μm.
Figure 2.
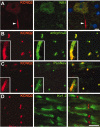
KCNQ2 is localized to CNS nodes and initial segments. These are images of sections of unfixed rat spinal cord, immunolabeled for KCNQ2 and NMDAR1 (A), ankyrin-G (B), Nav (C), or Kv1.2 (D); the merged image in A was also stained with the nuclear counterstain 4′,6′-diamidino-2-phenylindole. KCNQ2 labeling is concentrated in an initial segment (arrowhead) of an NMDAR1-positive motoneuron found in the ventral horn of spinal cord (A). KCNQ2 is colocalized with both ankyrin-G (B) and Nav (C) at nodes in the white matter and also at initial segments (B, C, insets are from motoneurons). By contrast, Kv1.2 is confined to the juxtaparanodes and does not colocalize with KCNQ2 (D). Scale bars: (including insets) 10 μm.
Figure 3.
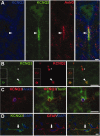
Distribution of KCNQ3 in the spinal cord. These are images of sections of unfixed rat spinal cord, immunostained as indicated. A, KCNQ3 staining surrounds the soma of a motoneuron in the ventral horn of the spinal cord but does not label the soma itself or the initial segment (arrowhead). B, Longitudinal section of the white matter stained for KCNQ3 (green) and KCNQ2 (red). Some KCNQ2-positive nodes are also KCNQ3 positive (arrowheads), whereas others are KCNQ3 negative (asterisks). Insets show transverse sections of both KCNQ3-positive (bottom) and KCNQ3-negative (top) nodes. C, Transverse section of the white matter stained for KCNQ3, ankyrin-G, and tenascin-R. KCNQ3 and tenascin-R appear to surround ankyrin-G-positive nodes. D, Transverse section of the white matter stained for KCNQ3 and GFAP, showing partially overlapping immunoreactivity in astrocytes (arrows). Scale bars: B, C, 5 μm; A, D, 10 μm.
Figure 5.
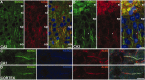
Colocalization of KCNQ2 and KCNQ3 in initial segments of cortex. These are images of horizontal sections of unfixed mouse brain immunolabeled for KCNQ2, KCNQ3, and ankyrin-G; DAPI was used as a nuclear counterstain in A and B. In the CA3 region of the hippocampus, many initial segments in the stratum pyramidale (sp), as well as the mossy fibers of the stratum lucidum (sl), are strongly KCNQ2 positive (A). The stratum radiatum (sr) and stratum oriens (so) are indicated. KCNQ3 was found with KCNQ2 in the initial segments of some pyramidal cells in CA3 but also in the mossy fibers (B). KCNQ3 colocalized with both ankyrin-G and KCNQ2 in the initial segment of neurons from the CA1 (C) and temporal neocortex (D). The asterisk in B marks a KCNQ3-positive blood vessel. Scale bars, 20 μm.
Figure 4.
Localization of KCNQ2 in the developing rat spinal cord. These are images of unfixed longitudinal sections of spinal cord from P4 (A), P8 (B), and P15 (C) rats, as well as P21 myelin-deficient rats (D), double labeled for KCNQ2 (red) and ankyrin-G or Nav (green). At P4 (A), few nodes (arrowheads) and no initial segments (insets) are KCNQ2 positive. At P8 (B), many nodes (arrowheads) and initial segments (insets) are KCNQ2 positive. At P15 (C), nearly all nodes and initial segments are KCNQ2 positive. In the spinal cord white matter of P21 myelin-deficient rats (D), where axons are undergoing demyelination because of oligodendrocytes cell death, KCNQ2 remained colocalized with Nav in node-like clusters. Scale bar: (including insets) 10 μm.
Figure 6.
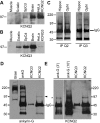
Immunoblots and immunoprecipitations. A, B, Immunoblot analysis. Membrane proteins (100 μg) from rat muscle, sciatic nerve, spinal cord, and brain, and HeLa celllysates (10 μg) were separated by electrophoresis and immunoblotted for KCNQ2 (A) or KCNQ3 (B). Bands corresponding to the molecular mass of KCNQ2 and KCNQ3 expressed in HeLa cells (∼97 kDa) were detected in both spinal cord and brain. KCNQ3, but not KCNQ2, was detected in sciatic nerve membrane. C, Immunoprecipitations of KCNQ2 and KCNQ3. Rat optic nerve and hippocampal membranes (200 μg) were immunoprecipitated for KCNQ2 and KCNQ3 and then immunoblotted with KCNQ2 or KCNQ3 antisera. KCNQ2 and KCNQ3 were detected in both samples. MW markers are shown on the left (in kilodaltons). D, E, Coimmunoprecipitations of KCNQ2 and ankyrin-G. Rat spinal cord membranes (200 μg) were immunoprecipitated for KCNQ2 or ankyrin-G and then immunoblotted for ankyrin-G (D) and KCNQ2 (E). A ∼97 kDa isoform of ankyrin-G was pulled down by the KCNQ2. The ankyrin-G antiserum pulled down multiple ankyrin-G isoforms, including the ∼97 kDa isoform. KCNQ2 (asterisk) was immunoprecipitated by both the ankyrin-G and the KCNQ2 antisera. MW markers are shown on the left (in kilodaltons). In E, the immunoblot for KCNQ2 is shown for two film exposure times: 3 min (3′) and 10 min (10′).
Figure 7.
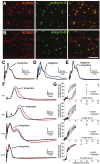
KCNQ2 modulates the excitability of premyelinated fibers. A, B, Images of horizontal sections of unfixed rat optic nerve immunolabeled for ankyrin-G and KCNQ2 (A) or KCNQ3 (B). Virtually all ankyrin-G nodes are strongly KCNQ2 positive, but a few are weakly positive for KCNQ3. Scale bar, 10 μm. C, E, CAPs recorded from 3-month-old rat optic nerves. Linopirdine (100 μ
m
) did not affect the CAP (n = 5) (C), but retigabine (20 μ
m
) delayed the onset of the CAP (n = 5) (D). The effects of retigabine were blocked by pretreating the nerves with linopirdine (20 μ
m
; n = 4) (E). F, G, CAPs recorded from P5, P11, P17, and 3-month-old rat optic nerves. Linopirdine (100 μ
m
) increased the duration (F) and refractory period (G) of the CAP at both P5 (n = 2) and P11 (n = 4) but not at P17 (n = 2) or 3 months (n = 5). For the refractory period, two stimuli are applied, and the amplitude of the highest peak of the second evoked CAP is measured.
Similar articles
- KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier.
Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, Kaji R, Bostock H. Schwarz JR, et al. J Physiol. 2006 May 15;573(Pt 1):17-34. doi: 10.1113/jphysiol.2006.106815. Epub 2006 Mar 9. J Physiol. 2006. PMID: 16527853 Free PMC article. - Kv3.1b is a novel component of CNS nodes.
Devaux J, Alcaraz G, Grinspan J, Bennett V, Joho R, Crest M, Scherer SS. Devaux J, et al. J Neurosci. 2003 Jun 1;23(11):4509-18. doi: 10.1523/JNEUROSCI.23-11-04509.2003. J Neurosci. 2003. PMID: 12805291 Free PMC article. - A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon.
Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. Pan Z, et al. J Neurosci. 2006 Mar 8;26(10):2599-613. doi: 10.1523/JNEUROSCI.4314-05.2006. J Neurosci. 2006. PMID: 16525039 Free PMC article. - Made for "anchorin": Kv7.2/7.3 (KCNQ2/KCNQ3) channels and the modulation of neuronal excitability in vertebrate axons.
Cooper EC. Cooper EC. Semin Cell Dev Biol. 2011 Apr;22(2):185-92. doi: 10.1016/j.semcdb.2010.10.001. Epub 2010 Oct 19. Semin Cell Dev Biol. 2011. PMID: 20940059 Free PMC article. Review. - KCNQ2/KCNQ3 K+ channels and the molecular pathogenesis of epilepsy: implications for therapy.
Rogawski MA. Rogawski MA. Trends Neurosci. 2000 Sep;23(9):393-8. doi: 10.1016/s0166-2236(00)01629-5. Trends Neurosci. 2000. PMID: 10941184 Review.
Cited by
- Phenotypic and functional assessment of two novel KCNQ2 gain-of-function variants Y141N and G239S and effects of amitriptyline treatment.
Bayat A, Iavarone S, Miceli F, Jakobsen AV, Johannesen KM, Nikanorova M, Ploski R, Szymanska K, Flamini R, Cooper EC, Weckhuysen S, Taglialatela M, Møller RS. Bayat A, et al. Neurotherapeutics. 2024 Jan;21(1):e00296. doi: 10.1016/j.neurot.2023.10.006. Epub 2023 Dec 19. Neurotherapeutics. 2024. PMID: 38241158 Free PMC article. - BetaIV spectrins are essential for membrane stability and the molecular organization of nodes of Ranvier.
Yang Y, Lacas-Gervais S, Morest DK, Solimena M, Rasband MN. Yang Y, et al. J Neurosci. 2004 Aug 18;24(33):7230-40. doi: 10.1523/JNEUROSCI.2125-04.2004. J Neurosci. 2004. PMID: 15317849 Free PMC article. - Selective interaction of syntaxin 1A with KCNQ2: possible implications for specific modulation of presynaptic activity.
Regev N, Degani-Katzav N, Korngreen A, Etzioni A, Siloni S, Alaimo A, Chikvashvili D, Villarroel A, Attali B, Lotan I. Regev N, et al. PLoS One. 2009 Aug 13;4(8):e6586. doi: 10.1371/journal.pone.0006586. PLoS One. 2009. PMID: 19675672 Free PMC article. - Function of KCNQ2 channels at nodes of Ranvier of lumbar spinal ventral nerves of rats.
Tonomura S, Ling J, Gu JG. Tonomura S, et al. Mol Brain. 2022 Jul 20;15(1):64. doi: 10.1186/s13041-022-00949-0. Mol Brain. 2022. PMID: 35858950 Free PMC article. - Cold aggravates abnormal excitability of motor axons in oxaliplatin-treated patients.
Bennedsgaard K, Ventzel L, Grafe P, Tigerholm J, Themistocleous AC, Bennett DL, Tankisi H, Finnerup NB. Bennedsgaard K, et al. Muscle Nerve. 2020 Jun;61(6):796-800. doi: 10.1002/mus.26852. Epub 2020 Mar 20. Muscle Nerve. 2020. PMID: 32133655 Free PMC article. Clinical Trial.
References
- Arroyo EJ, Scherer SS (2000) On the molecular architecture of myelinated fibers. Histochem Cell Biol 113: 1–18. - PubMed
- Arroyo EJ, Xu Y-T, Zhou L, Messing A, Peles E, Chiu SY, Scherer SS (1999) Myelinating Schwann cells determine the internodal localization of Kv1.1, Kv1.2, Kvβ2, and Caspr. J Neurocytol 28: 333–347. - PubMed
- Arroyo EJ, Xu T, Poliak S, Watson M, Peles E, Scherer SS (2001) Internodal specializations of myelinated axons in the CNS. Cell Tissue Res 305: 53–66. - PubMed
- Auger RG, Daube JR, Gomez MR, Lambert EH (1984) Hereditary form of sustained muscle activity of peripheral nerve origin causing generalized myokymia and muscle stiffness. Ann Neurol 15: 13–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases