Prostanoids, ornithine decarboxylase, and polyamines in primary chemoprevention of familial adenomatous polyposis - PubMed (original) (raw)
Clinical Trial
Prostanoids, ornithine decarboxylase, and polyamines in primary chemoprevention of familial adenomatous polyposis
Francis M Giardiello et al. Gastroenterology. 2004 Feb.
Abstract
Background & aims: Familial adenomatous polyposis because of germline mutation of the adenomatous polyposis coli gene is characterized by development of colorectal adenomas and, ultimately, colorectal cancer. The usefulness of colorectal mucosal compounds to predict the effect on adenoma development of primary chemoprevention with the nonsteroidal anti-inflammatory drug sulindac was evaluated.
Methods: A randomized, double-blind, placebo-controlled study of 41 subjects genotypically affected with familial adenomatous polyposis but phenotypically unaffected was conducted. Patients received either sulindac or placebo for 48 months, and development of new adenomas was evaluated. The levels of 5 prostanoids, ornithine decarboxylase, and polyamines were measured serially in normal-appearing rectal mucosa.
Results: There were no statistically significant differences between treatment groups in baseline levels of prostanoids, ornithine decarboxylase, or polyamines. At conclusion of the study, 4 of 5 prostaglandin levels were statistically significantly lower in the sulindac group than in the placebo group. Among the subset of patients taking sulindac, 3 of 5 prostaglandin levels were statistically significantly lower in patients who were polyp free than in those who developed polyps. By contrast, there were no statistically significant differences in ornithine decarboxylase or polyamines between treatment groups or in those on sulindac who were polyp free compared with those who developed polyps.
Conclusions: Colorectal mucosal prostaglandin levels, but not ornithine decarboxylase or polyamines, may be valuable biomarkers to assess appropriate drug dosage and medication compliance in patients undergoing primary chemoprevention therapy with sulindac. Reduction of mucosal prostaglandin levels may be necessary to achieve chemopreventive benefit from this agent.
Figures
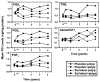
Figure 1
Serial mean prostaglandin levels (ng/mg/protein) in each of 4 patient groups: sulindac patients polyp free (◆), sulindac patients who developed polyps (■), placebo patients who are polyp free (▲), and placebo patients who developed polyps (●), for each of 5 prosglandin F2α [PGF2α], thromboxane B2 [TXB2], and 6 keto prostaglandin F1α [6KetoPGF1α]).
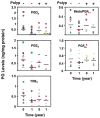
Figure 2
Individual patient prostaglandin levels (ng/mg protein) at baseline (time 0) and 1 year for patients in the sulindac group who were polyp free (−) and those who developed polyps (+) by study end for each of 5 prostaglandins (prostaglandin D2 [PGD2], prostaglandin E2 [PGE2], prostaglandin F2α [PGF2α], thromboxane B2 [TXB2], and 6 keto prostaglandin F1α [6KetoPGF1α]). *Indicates P < 0.005 and †indicates P < 0.05 when 1 year mean values are compared with baseline.
Similar articles
- Primary chemoprevention of familial adenomatous polyposis with sulindac.
Giardiello FM, Yang VW, Hylind LM, Krush AJ, Petersen GM, Trimbath JD, Piantadosi S, Garrett E, Geiman DE, Hubbard W, Offerhaus GJ, Hamilton SR. Giardiello FM, et al. N Engl J Med. 2002 Apr 4;346(14):1054-9. doi: 10.1056/NEJMoa012015. N Engl J Med. 2002. PMID: 11932472 Free PMC article. Clinical Trial. - Genetic polymorphisms of human flavin monooxygenase 3 in sulindac-mediated primary chemoprevention of familial adenomatous polyposis.
Hisamuddin IM, Wehbi MA, Chao A, Wyre HW, Hylind LM, Giardiello FM, Yang VW. Hisamuddin IM, et al. Clin Cancer Res. 2004 Dec 15;10(24):8357-62. doi: 10.1158/1078-0432.CCR-04-1073. Clin Cancer Res. 2004. PMID: 15623613 Clinical Trial. - [Chemoprevention of familial cancer].
Ishikawa H. Ishikawa H. Gan To Kagaku Ryoho. 1997 Jun;24(8):951-7. Gan To Kagaku Ryoho. 1997. PMID: 9212803 Review. Japanese. - Chemoprevention of carcinogenesis in familial tumors.
Ishikawa H. Ishikawa H. Int J Clin Oncol. 2004 Aug;9(4):299-303. doi: 10.1007/s10147-004-0417-1. Int J Clin Oncol. 2004. PMID: 15375706 Review.
Cited by
- In vitro and in vivo evaluation of NCX 4040 cytotoxic activity in human colon cancer cell lines.
Tesei A, Ulivi P, Fabbri F, Rosetti M, Leonetti C, Scarsella M, Zupi G, Amadori D, Bolla M, Zoli W. Tesei A, et al. J Transl Med. 2005 Feb 3;3(1):7. doi: 10.1186/1479-5876-3-7. J Transl Med. 2005. PMID: 15691389 Free PMC article. - Phase II study of the effects of ginger root extract on eicosanoids in colon mucosa in people at normal risk for colorectal cancer.
Zick SM, Turgeon DK, Vareed SK, Ruffin MT, Litzinger AJ, Wright BD, Alrawi S, Normolle DP, Djuric Z, Brenner DE. Zick SM, et al. Cancer Prev Res (Phila). 2011 Nov;4(11):1929-37. doi: 10.1158/1940-6207.CAPR-11-0224. Epub 2011 Oct 11. Cancer Prev Res (Phila). 2011. PMID: 21990307 Free PMC article. Clinical Trial. - Spectral biomarkers for chemoprevention of colonic neoplasia: a placebo-controlled double-blinded trial with aspirin.
Roy HK, Turzhitsky V, Wali R, Radosevich AJ, Jovanovic B, Della'Zanna G, Umar A, Rubin DT, Goldberg MJ, Bianchi L, De La Cruz M, Bogojevic A, Helenowski IB, Rodriguez L, Chatterton R, Skripkauskas S, Page K, Weber CR, Huang X, Richmond E, Bergan RC, Backman V. Roy HK, et al. Gut. 2017 Feb;66(2):285-292. doi: 10.1136/gutjnl-2015-309996. Epub 2015 Oct 26. Gut. 2017. PMID: 26503631 Free PMC article. Clinical Trial. - Chemoprevention in hereditary digestive neoplasia: A comprehensive review.
Chevalier E, Benamouzig R. Chevalier E, et al. Therap Adv Gastroenterol. 2023 Dec 1;16:17562848231215585. doi: 10.1177/17562848231215585. eCollection 2023. Therap Adv Gastroenterol. 2023. PMID: 38050626 Free PMC article. Review. - Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia.
Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C, Meyskens FL Jr, Brenner DE. Carroll RE, et al. Cancer Prev Res (Phila). 2011 Mar;4(3):354-64. doi: 10.1158/1940-6207.CAPR-10-0098. Cancer Prev Res (Phila). 2011. PMID: 21372035 Free PMC article. Clinical Trial.
References
- Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. - PubMed
- Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Babba S, Hedge P. Mutations of chromosome 5q21 gene in FAP and colorectal cancer patients. Science. 1991;253:665–669. - PubMed
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M. Identification and characterization of the Familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. - PubMed
- Joslyn G, Carlson M, Thliveris A, Albertsen H, Gelbert L, Samowitz W, Groden J, Stevens J, Spirio L, Robertson M. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991;66:600–613. - PubMed
- Bussey HJR. Familial polyposis coli. Family studies, histopathology, differential diagnosis, and results of treatment. Baltimore, Maryland: Johns Hopkins University Press; 1975.
Publication types
MeSH terms
Substances
Grants and funding
- CA 51085/CA/NCI NIH HHS/United States
- CA 53801/CA/NCI NIH HHS/United States
- R24 DK064399/DK/NIDDK NIH HHS/United States
- R24 DK064399-01/DK/NIDDK NIH HHS/United States
- CA 63721/CA/NCI NIH HHS/United States
- P50 CA 93-16/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources