Expression cloning of functional receptor used by SARS coronavirus - PubMed (original) (raw)
. 2004 Mar 5;315(2):439-44.
doi: 10.1016/j.bbrc.2004.01.076.
Jian Chen, Aihua Zheng, Yuchun Nie, Xuanling Shi, Wei Wang, Guangwen Wang, Min Luo, Huijun Liu, Lei Tan, Xijun Song, Zai Wang, Xiaolei Yin, Xiuxia Qu, Xiaojing Wang, Tingting Qing, Mingxiao Ding, Hongkui Deng
Affiliations
- PMID: 14766227
- PMCID: PMC7111169
- DOI: 10.1016/j.bbrc.2004.01.076
Expression cloning of functional receptor used by SARS coronavirus
Peigang Wang et al. Biochem Biophys Res Commun. 2004.
Abstract
We have expressed a series of truncated spike (S) glycoproteins of SARS-CoV and found that the N-terminus 14-502 residuals were sufficient to bind to SARS-CoV susceptible Vero E6 cells. With this soluble S protein fragment as an affinity ligand, we screened HeLa cells transduced with retroviral cDNA library from Vero E6 cells and obtained a HeLa cell clone which could bind with the S protein. This cell clone was susceptible to HIV/SARS pseudovirus infection and the presence of a functional receptor for S protein in this cell clone was confirmed by the cell-cell fusion assay. Further studies showed the susceptibility of this cell was due to the expression of endogenous angiotensin-converting enzyme 2 (ACE2) which was activated by inserted LTR from retroviral vector used for expression cloning. When human ACE2 cDNA was transduced into NIH3T3 cells, the ACE2 expressing NIH3T3 cells could be infected with HIV/SARS pseudovirus. These data clearly demonstrated that ACE2 was the functional receptor for SARS-CoV.
Figures
Fig. 1
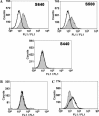
Soluble S1 proteins could bind with SARS-CoV susceptible Vero E6 cells. (A) S500 was sufficient to bind with Vero E6 cells. Binding of variously truncated S1 proteins to Vero E6 cells was measured by FACS using sera from SARS patient and a FITC-labeled anti-human IgG secondary antibody. Vero E6 cells were incubated with S1 conditioned mediums (shaded area) or culture medium (white). (B) Binding of S500 to HeLa cells was measured by FACS. HeLa cells were incubated with S500 conditioned medium (shaded area) or culture medium (white). (C) Pre-incubation of S500 with sera from SARS patient (shaded area) blocked the binding of S500 to Vero E6 cells.
Fig. 2
HIV/SARS susceptible clone F5 enriched from cDNA library transduced HeLa cells by FACS sorting with S500. (A) A HeLa cell clone with high binding affinity to S500 was enriched after five rounds of FACS sorting. In each round, the cells shown in gate were collected and cultured. (B) F5 cells and non-transduced HeLa cells were infected with HIV-luc/SARS pseudovirus and luciferase activity was measured after two days. The experiment was repeated for three times. (C) Syncytiums could be observed when F5 cells were co-cultured with S protein expressing HeLa cells (S-HeLa). Non-transduced HeLa cells were used as control and co-cultured with S-HeLa.
Fig. 3
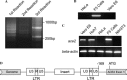
The insertion of LTR in F5 cells activated the expression of endogenous ACE2 gene. (A) After three TAIL-PCRs, a 900 bp band (top) and a 300 bp band (bottom) were detected by agarose gel. The PCR products were sequenced. The 900 bp PCR products consisted of a partial sequence of LTR and a partial sequence of the 5′ end of ACE2 gene. (B) A 300 bp band was detected in genomic DNA from F5 cells by PCR using primers specific to LTR and the ACE2 gene. Genomic DNAs from non-transduced HeLa cells and Vero E6 cells were used as control. (C) By RT-PCR, the expression of ACE2 was detected in Vero E6, Huh7, and F5 cells but not in non-transduced HeLa and NIH/3T3 cells. (D) Schematic representation of integration of LTR into the 5′ end of endogenous ACE2 gene. The start codon of ACE2 gene was 169 bp downstream to the 3′ LTR so that the ACE2 gene was under the transcriptional control of the 3′ LTR.
Fig. 4
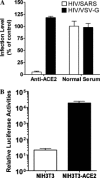
ACE2 mediated the entry of HIV/SARS pseudovirus. (A) The goat anti-human ACE2 polyclonal antibody could abrogate the entry of HIV-luc/SARS pseudovirus into F5 cells. Normal goat sera were used as control. (B) The ACE2 expressing NIH3T3 cells could be infected with HIV/SARS pseudovirus. NIH3T3 cells transduced with pMX vector were used as control.
Similar articles
- A study on antigenicity and receptor-binding ability of fragment 450-650 of the spike protein of SARS coronavirus.
Zhao J, Wang W, Yuan Z, Jia R, Zhao Z, Xu X, Lv P, Zhang Y, Jiang C, Gao XM. Zhao J, et al. Virology. 2007 Mar 15;359(2):362-70. doi: 10.1016/j.virol.2006.09.022. Epub 2006 Oct 20. Virology. 2007. PMID: 17055551 Free PMC article. - Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus.
Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Li W, et al. Nature. 2003 Nov 27;426(6965):450-4. doi: 10.1038/nature02145. Nature. 2003. PMID: 14647384 Free PMC article. - Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2.
Glende J, Schwegmann-Wessels C, Al-Falah M, Pfefferle S, Qu X, Deng H, Drosten C, Naim HY, Herrler G. Glende J, et al. Virology. 2008 Nov 25;381(2):215-21. doi: 10.1016/j.virol.2008.08.026. Epub 2008 Sep 23. Virology. 2008. PMID: 18814896 Free PMC article. - Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus.
Kuhn JH, Li W, Choe H, Farzan M. Kuhn JH, et al. Cell Mol Life Sci. 2004 Nov;61(21):2738-43. doi: 10.1007/s00018-004-4242-5. Cell Mol Life Sci. 2004. PMID: 15549175 Free PMC article. Review. - Insights from the association of SARS-CoV S-protein with its receptor, ACE2.
Li W, Choe H, Farzan M. Li W, et al. Adv Exp Med Biol. 2006;581:209-18. doi: 10.1007/978-0-387-33012-9_36. Adv Exp Med Biol. 2006. PMID: 17037532 Free PMC article. Review. No abstract available.
Cited by
- SARS-CoV, but not HCoV-NL63, utilizes cathepsins to infect cells: viral entry.
Huang IC, Bosch BJ, Li W, Farzan M, Rottier PM, Choe H. Huang IC, et al. Adv Exp Med Biol. 2006;581:335-8. doi: 10.1007/978-0-387-33012-9_60. Adv Exp Med Biol. 2006. PMID: 17037556 Free PMC article. No abstract available. - Retroviruses pseudotyped with the severe acute respiratory syndrome coronavirus spike protein efficiently infect cells expressing angiotensin-converting enzyme 2.
Moore MJ, Dorfman T, Li W, Wong SK, Li Y, Kuhn JH, Coderre J, Vasilieva N, Han Z, Greenough TC, Farzan M, Choe H. Moore MJ, et al. J Virol. 2004 Oct;78(19):10628-35. doi: 10.1128/JVI.78.19.10628-10635.2004. J Virol. 2004. PMID: 15367630 Free PMC article. - Identification and characterization of the putative fusion peptide of the severe acute respiratory syndrome-associated coronavirus spike protein.
Sainz B Jr, Rausch JM, Gallaher WR, Garry RF, Wimley WC. Sainz B Jr, et al. J Virol. 2005 Jun;79(11):7195-206. doi: 10.1128/JVI.79.11.7195-7206.2005. J Virol. 2005. PMID: 15890958 Free PMC article. - Coronaviral hypothetical and structural proteins were found in the intestinal surface enterocytes and pneumocytes of severe acute respiratory syndrome (SARS).
Chan WS, Wu C, Chow SC, Cheung T, To KF, Leung WK, Chan PK, Lee KC, Ng HK, Au DM, Lo AW. Chan WS, et al. Mod Pathol. 2005 Nov;18(11):1432-9. doi: 10.1038/modpathol.3800439. Mod Pathol. 2005. PMID: 15920543 Free PMC article. - Suppression of SARS-CoV entry by peptides corresponding to heptad regions on spike glycoprotein.
Yuan K, Yi L, Chen J, Qu X, Qing T, Rao X, Jiang P, Hu J, Xiong Z, Nie Y, Shi X, Wang W, Ling C, Yin X, Fan K, Lai L, Ding M, Deng H. Yuan K, et al. Biochem Biophys Res Commun. 2004 Jul 2;319(3):746-52. doi: 10.1016/j.bbrc.2004.05.046. Biochem Biophys Res Commun. 2004. PMID: 15184046 Free PMC article.
References
- Deng H.K., Unutmaz D., KewalRamani V.N., Littman D.R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. - PubMed
- Chan S.Y., Empig C.J., Welte F.J., Speck R.F., Schmaljohn A., Kreisberg J.F., Goldsmith M.A. Folate receptor-alpha is a cofactor for cellular entry by Marburg and Ebola viruses. Cell. 2001;106:117–126. - PubMed
- Pileri P., Uematsu Y., Campagnoli S., Galli G., Falugi F., Petracca R., Weiner A.J., Houghton M., Rosa D., Grandi G., Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. - PubMed
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous