A WD40 repeat protein regulates fungal cell differentiation and can be replaced functionally by the mammalian homologue striatin - PubMed (original) (raw)
A WD40 repeat protein regulates fungal cell differentiation and can be replaced functionally by the mammalian homologue striatin
Stefanie Pöggeler et al. Eukaryot Cell. 2004 Feb.
Abstract
Fruiting body development in fungi is a complex cellular differentiation process that is controlled by more than 100 developmental genes. Mutants of the filamentous fungus Sordaria macrospora showing defects in fruiting body formation are pertinent sources for the identification of components of this multicellular differentiation process. Here we show that the sterile mutant pro11 carries a defect in the pro11 gene encoding a multimodular WD40 repeat protein. Complementation analysis indicates that the wild-type gene or C-terminally truncated versions of the wild-type protein are able to restore the fertile phenotype in mutant pro11. PRO11 shows significant homology to several vertebrate WD40 proteins, such as striatin and zinedin, which seem to be involved in Ca2+-dependent signaling in cells of the central nervous system and are supposed to function as scaffolding proteins linking signaling and eukaryotic endocytosis. Cloning of a mouse cDNA encoding striatin allowed functional substitution of the wild-type protein with restoration of fertility in mutant pro11. Our data strongly suggest that an evolutionarily conserved cellular process controlling eukaryotic cell differentiation may regulate fruiting body formation.
Figures
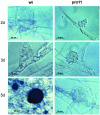
FIG. 1.
Sexual developmental stages of the wild-type (wt) and mutant (pro11) strains. Differential interference microscopy identified ascogonia (wild type, pro11), protoperithecia (wild type, pro11), and young perithecia (only wild-type strain). Strains were grown on fructification medium and examined after growth at 25°C for the number of days indicated.
FIG. 2.
Complementation analysis to restore fertility in pro11 and structural comparison of eukaryotic WD40 repeat proteins with PRO11 (AJ564211). (A) Molecular organization of the pro11 gene. Restriction map of the sequenced 4.0-kb fragment carrying the pro11 gene. The location of the open reading frame is indicated by an arrow, and the three introns are marked by grey boxes. Fragments generated by reverse transcription-PCR are shown below. (B) Recombinant plasmids carrying inserts of the pro11 gene were used for complementation experiments. The ability of transformants to restore fertility (wild-type phenotype) is indicated by a +, while no complementation is shown by −; (+) denotes transformants which produced few fertile perithecia but showed a hyphal morphology similar to that of the mutant strain. Abbreviations: A, _Apa_I; B, _Bam_HI; Bg, _Bgl_II; E, _Eco_RI; H, _Hin_dIII; P, _Pst_I; X, _Xho_I; Xb, _Xba_I. (C) Schematic representation of PRO11 and some closely related proteins. White boxes represent coiled-coil domains, and numbered black boxes show the position of WD40 repeats. Black boxes indicate the position of calmodulin binding sites and caveolin binding domains in striatin, SG2NA, and zinedin. Accession numbers: Mm, Mus musculus striatin (055106); Hs, Homo sapiens SG2NA (Q13033) and H. sapiens zinedin (NP_037535); Dm, Drosophila melanogaster CKA (Q9VLT9); Ce, Caenorhabditis elegans protein encoded by the K07C5.8 gene (Q17406); Sp, Schizosaccharomyces pombe protein encoded by the SPBC1773.01 gene (O94560). (D) Alignment of the seven predicted WD40 repeats in PRO11. Putative β strands are shaded and their positions are indicated with arrows (A to D). The WD40 repeat core consensus is given at the bottom. The coordinates of the amino acid positions are marked on the left.
FIG. 3.
Western blot analysis of polypeptides from different strains. Aliquots (30 μg) of protein fractions, as indicated, were fractionated on denaturing SDS-8% PAGE. Western blot detection of PRO11 protein was performed with a polyclonal PRO11 antibody. As a control, actin and porin were detected with specific antibodies in different protein fractions. Size markers are indicated. Abbreviations: wt, wild-type strain; pro11, pro11 mutant; Tr38, pro11 transformant carrying plasmid pIG1807-24; SF, soluble fraction; 100k, protein fractions as described in the text.
FIG. 4.
Ascus development in the wild type and a representative pro11 transformant (Tr) carrying plasmid pIG1808-23 and expressing the mouse striatin gene. (A) DAPI staining identified nuclei during karyogamy (K), meiosis II (MII), and postmeiotic mitosis (PM). The second postmeiotic mitosis (2nd PM) occurs after spore wall formation. (B) Rosettes from a mature perithecium are shown. Note that some of the asci of the transformant carry less than eight ascospores, which are regularly seen in a wild-type ascus.
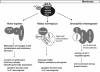
FIG. 5.
Scheme showing the signal transduction pathways of animal striatin-like proteins. All binding partners of the striatin-like proteins from mammals (Homo sapiens and Rattus norwegicus) and Drosophila melanogaster are shown in grey. With the exception of caveolin, genes encoding homologues for all depicted polypeptides have been identified in the fully sequenced genome of Neurospora crassa.
Similar articles
- Cell differentiation during sexual development of the fungus Sordaria macrospora requires ATP citrate lyase activity.
Nowrousian M, Masloff S, Pöggeler S, Kück U. Nowrousian M, et al. Mol Cell Biol. 1999 Jan;19(1):450-60. doi: 10.1128/MCB.19.1.450. Mol Cell Biol. 1999. PMID: 9858569 Free PMC article. - The phocein homologue SmMOB3 is essential for vegetative cell fusion and sexual development in the filamentous ascomycete Sordaria macrospora.
Bernhards Y, Pöggeler S. Bernhards Y, et al. Curr Genet. 2011 Apr;57(2):133-49. doi: 10.1007/s00294-010-0333-z. Epub 2011 Jan 13. Curr Genet. 2011. PMID: 21229248 Free PMC article. - Zinedin, SG2NA, and striatin are calmodulin-binding, WD repeat proteins principally expressed in the brain.
Castets F, Rakitina T, Gaillard S, Moqrich A, Mattei MG, Monneron A. Castets F, et al. J Biol Chem. 2000 Jun 30;275(26):19970-7. doi: 10.1074/jbc.M909782199. J Biol Chem. 2000. PMID: 10748158 - The composition and function of the striatin-interacting phosphatases and kinases (STRIPAK) complex in fungi.
Kück U, Beier AM, Teichert I. Kück U, et al. Fungal Genet Biol. 2016 May;90:31-38. doi: 10.1016/j.fgb.2015.10.001. Epub 2015 Oct 9. Fungal Genet Biol. 2016. PMID: 26439752 Review. - The filamentous fungus Sordaria macrospora as a genetic model to study fruiting body development.
Teichert I, Nowrousian M, Pöggeler S, Kück U. Teichert I, et al. Adv Genet. 2014;87:199-244. doi: 10.1016/B978-0-12-800149-3.00004-4. Adv Genet. 2014. PMID: 25311923 Review.
Cited by
- A high-throughput RNA-Seq approach to elucidate the transcriptional response of Piriformospora indica to high salt stress.
Nivedita, Rawoof A, Ramchiary N, Abdin MZ. Nivedita, et al. Sci Rep. 2021 Feb 18;11(1):4129. doi: 10.1038/s41598-021-82136-0. Sci Rep. 2021. PMID: 33602957 Free PMC article. - STRIPAK complexes: structure, biological function, and involvement in human diseases.
Hwang J, Pallas DC. Hwang J, et al. Int J Biochem Cell Biol. 2014 Feb;47:118-48. doi: 10.1016/j.biocel.2013.11.021. Epub 2013 Dec 11. Int J Biochem Cell Biol. 2014. PMID: 24333164 Free PMC article. Review. - In the Rat Midbrain, SG2NA and DJ-1 have Common Interactome, Including Mitochondrial Electron Transporters that are Comodulated Under Oxidative Stress.
Bisoyi P, Ratna D, Kumar G, Mallick BN, Goswami SK. Bisoyi P, et al. Cell Mol Neurobiol. 2023 Oct;43(7):3061-3080. doi: 10.1007/s10571-023-01356-2. Epub 2023 May 10. Cell Mol Neurobiol. 2023. PMID: 37165139 Review. - The nuclear Dbf2-related kinase COT1 and the mitogen-activated protein kinases MAK1 and MAK2 genetically interact to regulate filamentous growth, hyphal fusion and sexual development in Neurospora crassa.
Maerz S, Ziv C, Vogt N, Helmstaedt K, Cohen N, Gorovits R, Yarden O, Seiler S. Maerz S, et al. Genetics. 2008 Jul;179(3):1313-25. doi: 10.1534/genetics.108.089425. Epub 2008 Jun 18. Genetics. 2008. PMID: 18562669 Free PMC article. - Analysis of the Putative Nucleoporin POM33 in the Filamentous Fungus Sordaria macrospora.
Groth A, Schmitt K, Valerius O, Herzog B, Pöggeler S. Groth A, et al. J Fungi (Basel). 2021 Aug 24;7(9):682. doi: 10.3390/jof7090682. J Fungi (Basel). 2021. PMID: 34575720 Free PMC article.
References
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
- Baillat, G., S. Gaillard, F. Castets, and A. Monneron. 2002. Interactions of phocein with nucleoside-diphosphate kinase, Eps15, and Dynamin I. J. Biol. Chem. 277:18961-18966. - PubMed
- Bartoli, M., A. Monneron, and D. Ladant. 1998. Interaction of calmodulin with striatin, a WD-repeat protein present in neuronal dendritic spines. J. Biol. Chem. 273:22248-22253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous