Dopamine operates as a subsecond modulator of food seeking - PubMed (original) (raw)
Dopamine operates as a subsecond modulator of food seeking
Mitchell F Roitman et al. J Neurosci. 2004.
Abstract
The dopamine projection to the nucleus accumbens has been implicated in behaviors directed toward the acquisition and consumption of natural rewards. The neurochemical studies that established this link made time-averaged measurements over minutes, and so the precise temporal relationship between dopamine changes and these behaviors is not known. To resolve this, we sampled dopamine every 100 msec using fast-scan cyclic voltammetry at carbon-fiber microelectrodes in the nucleus accumbens of rats trained to press a lever for sucrose. Cues that signal the opportunity to respond for sucrose evoked dopamine release (67 +/- 20 nm) with short latency (0.2 +/- 0.1 sec onset). When the same cues were presented to rats naive to the cue-sucrose pairing, similar dopamine signals were not observed. Thus, cue-evoked increases in dopamine in trained rats reflected a learned association between the cues and sucrose availability. Lever presses for sucrose occurred at the peak of the dopamine surges. After lever presses, and while sucrose was delivered and consumed, no further increases in dopamine were detected. Rather, dopamine returned to baseline levels. Together, the results strongly implicate subsecond dopamine signaling in the nucleus accumbens as a real-time modulator of food-seeking behavior.
Figures
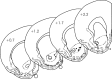
Figure 1.
Histological verification of recording sites. Electrolytic lesions confirmed that recording sites (•) were within the nucleus accumbens core. The numbers on individual sections indicate distance, in millimeters, anterior to bregma (Paxinos and Watson, 1998).
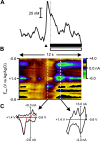
Figure 2.
Dopamine release is evoked by stimulation of the ventral tegmental area. The electrochemical data obtained in the nucleus accumbens during a representative electrical stimulation is shown in the color plot (A). The voltammetric current (represented in color) is plotted against the applied potential (_E_app; ordinate) for each scan. Consecutive scans (every 100 msec) are represented along the abscissa (time). Dopamine increased during the stimulation (24 biphasic pulses, 60 Hz, ±120 μA; red bar). It was identified from its peaks at 0.67 and -0.20 V. The oxidation peak (green) is accompanied by its reduction peak (yellow). The cyclic voltammogram (current-voltage plot) (B, left) extracted from these data are similar to exogenous dopamine in a flow-cell apparatus. The events that take place in the later part of the color plot, and are shown in the right-hand cyclic voltammogram (B, right), are clearly not changes in dopamine concentration but more likely a basic pH change in the extracellular space (Venton et al., 2003). The time courses of electrochemical signals taken at different applied potentials are shown in C: x is the current (vs time) at the peak oxidation potential for dopamine (derived from the current-voltage plot), and y is the current at a potential insufficient to oxidize dopamine. Scaling y to the sensitivity at x and subtracting it removes the non-dopaminergic interference from the signal. This is then converted to dopamine concentration by normalization to an in vitro calibration of known dopamine concentration. The red boxes highlight the data points at which electrical stimulation began and ended.
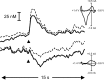
Figure 3.
Cue presentation elicits a phasic surge in dopamine. A, The rise in signal evoked by a representative cue presentation (denoted by black triangle). The peak in dopamine concentration occurred just before the operant response for intra-oral sucrose (denoted by vertical dashed line) and fell to baseline levels during the duration of the intra-oral infusion (black bar) and tone-light stimulus (open bar). B, Dopamine was identified from its peaks at 0.66 and -0.22 V and in the cyclic voltammogram (C, left). As can be seen in the color plot, the oxidation peak (green) is accompanied by its reduction peak (yellow). A statistical comparison of this cyclic voltammogram with one from electrically evoked dopamine release (normalized and shown in red) reveals a close correlation (_r_2 = 0.77). The events that take place in the later part of the color plot, and shown in the right-hand cyclic voltammogram (C, right), are clearly not changes in dopamine concentration. This cyclic voltammogram was not correlated with the one from electrically evoked dopamine release (_r_2 = 0.16). Rather, the events in the latter part of the color plot are more likely caused by a basic pH change in the extracellular space. This interference was removed from the signal trace in the same manner as described for removing interference from electrically evoked dopamine release (Fig. 2).
Figure 4.
Cue presentation evokes phasic surges in dopamine and reflects a learned association. Increases in signal (mean + SEM represented by solid and dashed black lines, respectively) were evoked by cue presentation (denoted by black triangle) for operant responding rats (n = 5; top trace). The robust increase in signal was confirmed to be a rise in dopamine concentration by examination of the averaged cyclic voltammogram taken at the peak of the signal (inset). Cue presentation failed to elicit an increase in dopamine in rats (n = 3; bottom trace) that did not have the cue-sucrose pairing. The decrease in signal just after cue presentation was not caused by a change in dopamine. This was confirmed by comparison of the averaged cyclic voltammogram (inset) with the averaged cyclic voltammogram taken from pre-session, electrically evoked dopamine release (_r_2 < 0.01). The signal in operant responding rats was significantly higher than control rats immediately after cue presentation (0.5-2.4 sec; p < 0.05; post hoc Student's t test with Bonferroni correction).
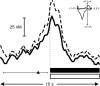
Figure 5.
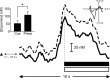
Rats lever press at the peak of cue-evoked dopamine release. Increases in signal (mean + SEM represented by solid and dashed black lines, respectively) began immediately before and peaked at the operant response (denoted by vertical dashed line) on trials when cue presentation elicited an immediate behavioral response (<5 sec latency to press). The average time of cue presentation is denoted by the black triangle, and the range of times is represented by the horizontal scale bar. The increase in signal at the time of the lever press was confirmed to be dopamine by comparison of the averaged cyclic voltammogram taken at the peak of the signal (inset) with the averaged cyclic voltammogram from pre-session electrically evoked dopamine release (_r_2 = 0.90). Dopamine concentration rapidly returned to baseline levels during sucrose infusion (horizontal black bar) and tone-light presentation (horizontal open bar) and remained stable thereafter.
Figure 6.
Even when cues fail to elicit an immediate behavioral response, rats lever press at a dopamine peak. A dramatic increase in signal (mean + SEM represented by solid and dashed black lines, respectively) was observed immediately before and peaked at the operant response (denoted by vertical dashed line) on trials when cue presentation failed to elicit an immediate behavioral response (>5 sec latency to press). The cue presentation (black triangle) was at a time (0.1-133.4 sec) before the start of the trace. Cue presentation elicited a rise in dopamine, but rats did not press the lever until a second, larger rise in dopamine occurred (*p < 0.05; left inset). The large rise in signal was confirmed to be dopamine release by comparison of averaged cyclic voltammogram (right inset) taken from the peak of the signal with that from pre-session, electrically evoked dopamine release (_r_2 = 0.87). Dopamine concentration returned to baseline levels during sucrose infusion (horizontal black bar) and tone-light presentation (horizontal open bar).
Similar articles
- The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues.
Yun IA, Wakabayashi KT, Fields HL, Nicola SM. Yun IA, et al. J Neurosci. 2004 Mar 24;24(12):2923-33. doi: 10.1523/JNEUROSCI.5282-03.2004. J Neurosci. 2004. PMID: 15044531 Free PMC article. - Nucleus accumbens dopamine and mu-opioid receptors modulate the reinstatement of food-seeking behavior by food-associated cues.
Guy EG, Choi E, Pratt WE. Guy EG, et al. Behav Brain Res. 2011 Jun 1;219(2):265-72. doi: 10.1016/j.bbr.2011.01.024. Epub 2011 Jan 22. Behav Brain Res. 2011. PMID: 21262268 - Differential dopamine release dynamics in the nucleus accumbens core and shell track distinct aspects of goal-directed behavior for sucrose.
Cacciapaglia F, Saddoris MP, Wightman RM, Carelli RM. Cacciapaglia F, et al. Neuropharmacology. 2012 Apr;62(5-6):2050-6. doi: 10.1016/j.neuropharm.2011.12.027. Epub 2012 Jan 12. Neuropharmacology. 2012. PMID: 22261383 Free PMC article. - Nucleus accumbens cell firing and rapid dopamine signaling during goal-directed behaviors in rats.
Carelli RM. Carelli RM. Neuropharmacology. 2004;47 Suppl 1:180-9. doi: 10.1016/j.neuropharm.2004.07.017. Neuropharmacology. 2004. PMID: 15464136 Review. - Environmental enrichment reduces food seeking and taking in rats: A review.
Grimm JW, Sauter F. Grimm JW, et al. Pharmacol Biochem Behav. 2020 Mar;190:172874. doi: 10.1016/j.pbb.2020.172874. Epub 2020 Feb 19. Pharmacol Biochem Behav. 2020. PMID: 32084492 Free PMC article. Review.
Cited by
- Dopamine release plateau and outcome signals in dorsal striatum contrast with classic reinforcement learning formulations.
Kim MJ, Gibson DJ, Hu D, Yoshida T, Hueske E, Matsushima A, Mahar A, Schofield CJ, Sompolpong P, Tran KT, Tian L, Graybiel AM. Kim MJ, et al. Nat Commun. 2024 Oct 14;15(1):8856. doi: 10.1038/s41467-024-53176-7. Nat Commun. 2024. PMID: 39402067 Free PMC article. - The nucleus accumbens in reward and aversion processing: insights and implications.
Xu Y, Lin Y, Yu M, Zhou K. Xu Y, et al. Front Behav Neurosci. 2024 Aug 9;18:1420028. doi: 10.3389/fnbeh.2024.1420028. eCollection 2024. Front Behav Neurosci. 2024. PMID: 39184934 Free PMC article. Review. - Kv7 channel opener retigabine reduces self-administration of cocaine but not sucrose in rats.
Urena ES, Diezel CC, Serna M, Hala'ufia G, Majuta L, Barber KR, Vanderah TW, Riegel AC. Urena ES, et al. Addict Biol. 2024 Aug;29(8):e13428. doi: 10.1111/adb.13428. Addict Biol. 2024. PMID: 39087789 Free PMC article. - Constraints on the subsecond modulation of striatal dynamics by physiological dopamine signaling.
Long C, Lee K, Yang L, Dafalias T, Wu AK, Masmanidis SC. Long C, et al. Nat Neurosci. 2024 Oct;27(10):1977-1986. doi: 10.1038/s41593-024-01699-z. Epub 2024 Jul 3. Nat Neurosci. 2024. PMID: 38961230 - Hypocretin-1 receptor antagonism improves inhibitory control during the Go/No-Go task in highly motivated, impulsive male mice.
Metha J, Ji Y, Braun C, Nicholson JR, De Lecea L, Murawski C, Hoyer D, Jacobson LH. Metha J, et al. Psychopharmacology (Berl). 2024 Oct;241(10):2171-2187. doi: 10.1007/s00213-024-06628-3. Epub 2024 Jun 18. Psychopharmacology (Berl). 2024. PMID: 38886189 Free PMC article.
References
- Bassareo V, Di Chiara G (1999) Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience 89: 637-641. - PubMed
- Berridge CW, Stratford TL, Foote SL, Kelley AE (1997) Distribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse 27: 230-241. - PubMed
- Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28: 309-369. - PubMed
- Carelli RM (2002) Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. “natural” reinforcement. Physiol Behav 76: 379-387. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources