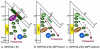Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1 - PubMed (original) (raw)
Review
Role of ICP0 in the strategy of conquest of the host cell by herpes simplex virus 1
Ryan Hagglund et al. J Virol. 2004 Mar.
No abstract available
Figures
FIG. 1.
Models showing interactions of ICP0 with the ubiquitin-proteasome system. (A) HUL-1 E3 domain. ICP0 HUL-1 E3 activity, mapping in sequences encoded in exon 3, promotes the autoubiquitylation and degradation of the E2 enzyme cdc34 (62-64, 152). The model depicted is described in the text. The figure is adapted from reference . (B) RING finger (HUL-2) E3 domain mapping to sequences encoded by exon 2. ICP0 RING finger E3 activity promotes the ubiquitylation and degradation of PML and other cellular proteins (13, 45, 46, 95, 120, 122). While the ICP0 RING finger has E3 ligase activity in in vitro reactions containing either UbcH5a or UbcH6 E2 ubiquitin-conjugating enzymes (14, 64), the degradation of PML and Sp100 appears to be mediated by UbcH5a (60). The E2 enzyme(s) involved in the degradation of the other known substrates of the ICP0 RING finger has not been identified. Available data indicate that ICP0 binds USP7 and sequesters it from deubiquitylating substrates ubiquitylated by ICP0. (C) Model of HUL-2 E3 in cells infected with a mutant incapable of binding USP7. The model shows that unbound USP7 would be available to deubiquitylate newly ubiquitylated ICP0 substrates, decreasing the efficiency of their degradation. This model is consistent with data showing that mutants unable to bind USP7 have a reduced capacity to degrade PML and Sp100 (; Hagglund and Roizman, unpublished).
Similar articles
- Sequences related to SUMO interaction motifs in herpes simplex virus 1 protein ICP0 act cooperatively to stimulate virus infection.
Everett RD, Boutell C, Pheasant K, Cuchet-Lourenço D, Orr A. Everett RD, et al. J Virol. 2014 Mar;88(5):2763-74. doi: 10.1128/JVI.03417-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352468 Free PMC article. - Cellular Transcriptional Coactivator RanBP10 and Herpes Simplex Virus 1 ICP0 Interact and Synergistically Promote Viral Gene Expression and Replication.
Sato Y, Kato A, Maruzuru Y, Oyama M, Kozuka-Hata H, Arii J, Kawaguchi Y. Sato Y, et al. J Virol. 2016 Jan 6;90(6):3173-86. doi: 10.1128/JVI.03043-15. J Virol. 2016. PMID: 26739050 Free PMC article. - Analysis of the functional interchange between the IE1 and pp71 proteins of human cytomegalovirus and ICP0 of herpes simplex virus 1.
Lu Y, Everett RD. Lu Y, et al. J Virol. 2015 Mar;89(6):3062-75. doi: 10.1128/JVI.03480-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552717 Free PMC article. - Two overlapping regions within the N-terminal half of the herpes simplex virus 1 E3 ubiquitin ligase ICP0 facilitate the degradation and dissociation of PML and dissociation of Sp100 from ND10.
Perusina Lanfranca M, Mostafa HH, Davido DJ. Perusina Lanfranca M, et al. J Virol. 2013 Dec;87(24):13287-96. doi: 10.1128/JVI.02304-13. Epub 2013 Oct 2. J Virol. 2013. PMID: 24089549 Free PMC article. - The potential link between PML NBs and ICP0 in regulating lytic and latent infection of HSV-1.
Wang S, Long J, Zheng CF. Wang S, et al. Protein Cell. 2012 May;3(5):372-82. doi: 10.1007/s13238-012-2021-x. Epub 2012 Apr 28. Protein Cell. 2012. PMID: 22544561 Free PMC article. Review.
Cited by
- Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100.
Everett RD, Parada C, Gripon P, Sirma H, Orr A. Everett RD, et al. J Virol. 2008 Mar;82(6):2661-72. doi: 10.1128/JVI.02308-07. Epub 2007 Dec 26. J Virol. 2008. PMID: 18160441 Free PMC article. - Sequences related to SUMO interaction motifs in herpes simplex virus 1 protein ICP0 act cooperatively to stimulate virus infection.
Everett RD, Boutell C, Pheasant K, Cuchet-Lourenço D, Orr A. Everett RD, et al. J Virol. 2014 Mar;88(5):2763-74. doi: 10.1128/JVI.03417-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352468 Free PMC article. - L Particles Transmit Viral Proteins from Herpes Simplex Virus 1-Infected Mature Dendritic Cells to Uninfected Bystander Cells, Inducing CD83 Downmodulation.
Heilingloh CS, Kummer M, Mühl-Zürbes P, Drassner C, Daniel C, Klewer M, Steinkasserer A. Heilingloh CS, et al. J Virol. 2015 Nov;89(21):11046-55. doi: 10.1128/JVI.01517-15. Epub 2015 Aug 26. J Virol. 2015. PMID: 26311871 Free PMC article. - A CRISPR-based rapid DNA repositioning strategy and the early intranuclear life of HSV-1.
Xiang J, Fan C, Dong H, Ma Y, Xu P. Xiang J, et al. Elife. 2023 Sep 13;12:e85412. doi: 10.7554/eLife.85412. Elife. 2023. PMID: 37702383 Free PMC article. - Mutational inactivation of herpes simplex virus 1 microRNAs identifies viral mRNA targets and reveals phenotypic effects in culture.
Flores O, Nakayama S, Whisnant AW, Javanbakht H, Cullen BR, Bloom DC. Flores O, et al. J Virol. 2013 Jun;87(12):6589-603. doi: 10.1128/JVI.00504-13. Epub 2013 Mar 27. J Virol. 2013. PMID: 23536669 Free PMC article.
References
- Advani, S. J., R. Brandimarti, R. R. Weichselbaum, and B. Roizman. 2000. The disappearance of cyclins A and B and the increase in activity of the G2/M-phase cellular kinase cdc2 in herpes simplex virus 1-infected cells require expression of the α22/US1.5 and UL13 viral genes. J. Virol. 74:8-15. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA78766/CA/NCI NIH HHS/United States
- CA83939/CA/NCI NIH HHS/United States
- R01 CA078766/CA/NCI NIH HHS/United States
- CA71933/CA/NCI NIH HHS/United States
- CA87661/CA/NCI NIH HHS/United States
- P01 CA087661/CA/NCI NIH HHS/United States
- P01 CA071933/CA/NCI NIH HHS/United States
- R01 CA088860/CA/NCI NIH HHS/United States
- CA88860/CA/NCI NIH HHS/United States
- R37 CA078766/CA/NCI NIH HHS/United States
- R01 CA083939/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources