Dilated cardiomyopathy caused by tissue-specific ablation of SC35 in the heart - PubMed (original) (raw)
. 2004 Feb 25;23(4):885-96.
doi: 10.1038/sj.emboj.7600054. Epub 2004 Feb 12.
Xiangdong Xu, Dongmei Yang, Pao-Hsien Chu, Nancy D Dalton, Zhen Ye, Joanne M Yeakley, Heping Cheng, Rui-Ping Xiao, John Ross, Ju Chen, Xiang-Dong Fu
Affiliations
- PMID: 14963485
- PMCID: PMC380988
- DOI: 10.1038/sj.emboj.7600054
Dilated cardiomyopathy caused by tissue-specific ablation of SC35 in the heart
Jian-Hua Ding et al. EMBO J. 2004.
Abstract
Many genetic diseases are caused by mutations in cis-acting splicing signals, but few are triggered by defective trans-acting splicing factors. Here we report that tissue-specific ablation of the splicing factor SC35 in the heart causes dilated cardiomyopathy (DCM). Although SC35 was deleted early in cardiogenesis by using the MLC-2v-Cre transgenic mouse, heart development appeared largely unaffected, with the DCM phenotype developing 3-5 weeks after birth and the mutant animals having a normal life span. This nonlethal phenotype allowed the identification of downregulated genes by microarray, one of which was the cardiac-specific ryanodine receptor 2. We showed that downregulation of this critical Ca2+ release channel preceded disease symptoms and that the mutant cardiomyocytes exhibited frequency-dependent excitation-contraction coupling defects. The implication of SC35 in heart disease agrees with a recently documented link of SC35 expression to heart failure and interference of splicing regulation during infection by myocarditis-causing viruses. These studies raise a new paradigm for the etiology of certain human heart diseases of genetic or environmental origin that may be triggered by dysfunction in RNA processing.
Figures
Figure 1
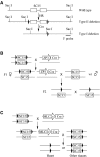
Germline and ventricle-specific deletion of SC35. (A) Map of the floxed SC35 locus generated by homologous recombination (HR) and Cre-mediated conversion. Generation of floxed SC35 mouse (type II deletion) was as previously described (Wang et al, 2001). Key SacI sites are illustrated and the 3′ probe was used to discriminate among wild type, type II and type I deletions. (B) Strategy to generate SC35 knockout in the germline. The zona pellucida 3 (ZP3) gene is expressed exclusively in the primary oocyte prior to the completion of the first meiotic division. After crossing floxed homozygous SC35 mice with ZP3-Cre transgenic mouse, the F1 female floxed SC35/Cre+ progeny were then mated with wild-type male mice to generate heterozygous SC35 type I deletions in the germline. Heterozygous SC35 knockout mice were mated with each other to produce homozygous knockout animals. (C) Strategy to generate ventricle-specific SC35 knockout. The MLC-2v promoter is turned on to express Cre at E8.5. After crossing floxed homozygous SC35 mice with the MLC2v-Cre knockin mouse, the double heterozygous floxed SC35/Cre+ mice were then crossed with homozygous floxed SC35 mice. This cross should generate 25% Cre+ mice in which SC35 was specifically ablated in the heart.
Figure 2
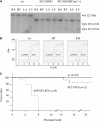
Nonlethal phenotype with SC35 ablation in the heart. (A) Specificity and efficiency of MLC2v-Cre-mediated recombination in the heart. Genomic DNA from right atrium (RA), left atrium (LA), right ventricle (RV) and left ventricle (LV) were digested with SacI and analyzed by Southern blotting. Recombination is restricted to the ventricles. (B) Quantitative PCR analysis of the SC35 transcript. The GAPDH transcript was analyzed as a control. (C) Mortality curve for SC35 ablation in the heart. Over 80% SC35-deficient mice have a normal life span similar to their littermates in the same cage. In contrast, all similarly targeted SF2/ASF mice died within 8 weeks after birth.
Figure 3
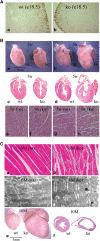
Histological and pathological analysis of SC35-deficient hearts. (A) Cell proliferation assay by BrdU labeling. Mice were injected with BrdU at E18.5 and ventricles were isolated 22 h later. Paraffin sections of ventricles were blotted with biotinylated anti-BrdU antibody followed by staining with HRP-conjugated streptavidin in the presence of the substrate AEC. Proliferating cells were stained as brown dots in the periphery of the heart. (B) Isolated mutant hearts and their littermate controls at 3 and 5 weeks after birth (a and b); H&E-stained coronal sections showing normal cardiac histology 3 weeks after birth, but chamber enlargement and cardiomyocyte hypertrophy at postnatal 5 weeks (c–h). (C) Extensive fibrosis and myofibril disarray in SC35-deficient hearts were evident at an advanced stage (a and b). However, the sarcomere structure appeared normal under the electron microscope (c and d). Dramatic heart enlargement and chamber dilation were seen with the SC35 knockout hearts at late stages (c and d). The stages (in months) indicate the time when the hearts were collected for analysis, which do not represent the phenotype onset points.
Figure 4
Profiling gene expression in SC35-ablated ventricles. Total RNA was extracted from SC35-deficient ventricles and littermate controls, converted to cRNA, and hybridized to Affymetrix U74Av2 chips. Results are from three independent experiments for both wild-type and mutant mice. Fold changes (also highlighted by gray degree), gene ID, and names are listed.
Figure 5
Molecular characterization of SC35-deficient hearts. (A) Northern blotting analysis of both hypertrophy markers and critical components of the troponin complex in wild-type, hetero-, and homozygous SC35-ablated mice. Note that an apparent elevation of TnI expression in a heterozygous heart was not a reproducible result. (B) Quantitative PCR analysis of RyR2 RNA at two different developmental stages (E19.5 and postnatal 1 month). GAPDH was analyzed as a control. Each experiment was duplicated. (C) Western blotting analysis of RyR2 protein in an sarcoplasmic reticulum-enriched cellular fraction. The sarcoplasmic reticulum resident marker calsequestrin was analyzed as a loading control.
Figure 6
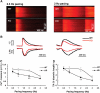
Deficient EC coupling in SC35 knockout cardiomyocytes. (A) Typical confocal Ca2+ images of single isolated cardiomyocytes from wild-type or knockout mice. Cultured cells were loaded with fluo-4 followed by pacing at designated frequencies. Line-scan images are displayed with time and space on the abscissa and ordinate, respectively. (B) Traces of spatially averaged Ca2+ transients (top) and the corresponding cell shortenings (bottom, downward defections). (C) Frequency dependence of peak Ca2+ transient (Δ_F/F_0, where _F_0 refers to the fluo-4 signal at rest). (D) Frequency dependence of twitch amplitude (TA, presented as percent diastolic cell length). *P<0.05; **P<0.01 by Student's _t_-test. _N_=17–22 for each data point.
Similar articles
- Cardiomyocyte-Specific Ablation of Med1 Subunit of the Mediator Complex Causes Lethal Dilated Cardiomyopathy in Mice.
Jia Y, Chang HC, Schipma MJ, Liu J, Shete V, Liu N, Sato T, Thorp EB, Barger PM, Zhu YJ, Viswakarma N, Kanwar YS, Ardehali H, Thimmapaya B, Reddy JK. Jia Y, et al. PLoS One. 2016 Aug 22;11(8):e0160755. doi: 10.1371/journal.pone.0160755. eCollection 2016. PLoS One. 2016. PMID: 27548259 Free PMC article. - Gene expression profile in dilated cardiomyopathy caused by elevated frequencies of mitochondrial DNA mutations in the mouse heart.
Zhang D, Ezekiel UR, Chang SW, Zassenhaus HP. Zhang D, et al. Cardiovasc Pathol. 2005 Mar-Apr;14(2):61-9. doi: 10.1016/j.carpath.2005.01.006. Cardiovasc Pathol. 2005. PMID: 15780797 - Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity.
Rajan S, Ahmed RP, Jagatheesan G, Petrashevskaya N, Boivin GP, Urboniene D, Arteaga GM, Wolska BM, Solaro RJ, Liggett SB, Wieczorek DF. Rajan S, et al. Circ Res. 2007 Jul 20;101(2):205-14. doi: 10.1161/CIRCRESAHA.107.148379. Epub 2007 Jun 7. Circ Res. 2007. PMID: 17556658 - Inflammatory dilated cardiomyopathy (DCMI).
Maisch B, Richter A, Sandmöller A, Portig I, Pankuweit S; BMBF-Heart Failure Network. Maisch B, et al. Herz. 2005 Sep;30(6):535-44. doi: 10.1007/s00059-005-2730-5. Herz. 2005. PMID: 16170686 Review. - Mechanisms of RBM20 Cardiomyopathy: Insights From Model Systems.
Gregorich ZR, Zhang Y, Kamp TJ, Granzier HL, Guo W. Gregorich ZR, et al. Circ Genom Precis Med. 2024 Feb;17(1):e004355. doi: 10.1161/CIRCGEN.123.004355. Epub 2024 Jan 30. Circ Genom Precis Med. 2024. PMID: 38288598 Review.
Cited by
- The alternative heart: impact of alternative splicing in heart disease.
Lara-Pezzi E, Gómez-Salinero J, Gatto A, García-Pavía P. Lara-Pezzi E, et al. J Cardiovasc Transl Res. 2013 Dec;6(6):945-55. doi: 10.1007/s12265-013-9482-z. Epub 2013 Jun 18. J Cardiovasc Transl Res. 2013. PMID: 23775418 Review. - SRSF2 Is Essential for Hematopoiesis, and Its Myelodysplastic Syndrome-Related Mutations Dysregulate Alternative Pre-mRNA Splicing.
Komeno Y, Huang YJ, Qiu J, Lin L, Xu Y, Zhou Y, Chen L, Monterroza DD, Li H, DeKelver RC, Yan M, Fu XD, Zhang DE. Komeno Y, et al. Mol Cell Biol. 2015 Sep 1;35(17):3071-82. doi: 10.1128/MCB.00202-15. Epub 2015 Jun 29. Mol Cell Biol. 2015. PMID: 26124281 Free PMC article. - The impact of alternative splicing in vivo: mouse models show the way.
Möröy T, Heyd F. Möröy T, et al. RNA. 2007 Aug;13(8):1155-71. doi: 10.1261/rna.554607. Epub 2007 Jun 11. RNA. 2007. PMID: 17563071 Free PMC article. Review. - A new function of the splicing factor SRSF2 in the control of E2F1-mediated cell cycle progression in neuroendocrine lung tumors.
Edmond V, Merdzhanova G, Gout S, Brambilla E, Gazzeri S, Eymin B. Edmond V, et al. Cell Cycle. 2013 Apr 15;12(8):1267-78. doi: 10.4161/cc.24363. Epub 2013 Mar 21. Cell Cycle. 2013. PMID: 23518498 Free PMC article. - Global impact of RNA splicing on transcriptome remodeling in the heart.
Gao C, Wang Y. Gao C, et al. J Zhejiang Univ Sci B. 2012 Aug;13(8):603-8. doi: 10.1631/jzus.B1201006. J Zhejiang Univ Sci B. 2012. PMID: 22843179 Free PMC article. Review.
References
- Biesiadecki BJ, Elder BD, Yu ZB, Jin JP (2002) Cardiac troponin T variants produced by aberrant splicing of multiple exons in animals with high instances of dilated cardiomyopathy. J Biol Chem 277: 50275–50285 - PubMed
- Black DL (2000) Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell 103: 367–370 - PubMed
- Bonne G, Carrier L, Richard P, Hainque B, Schwartz K (1998) Familial hypertrophic cardiomyopathy: from mutations to functional defects. Circ Res 83: 580–593 - PubMed
- Burge CB, Tuschl TH, Sharp PA (1999) Splicing of precursors to mRNA by the spliceosome. In The RNA World II, Gesteland RF, Cech TR, Atkins JF (eds), pp 525–560
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous