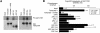Functional subdomain in the ankyrin domain of tankyrase 1 required for poly(ADP-ribosyl)ation of TRF1 and telomere elongation - PubMed (original) (raw)
Functional subdomain in the ankyrin domain of tankyrase 1 required for poly(ADP-ribosyl)ation of TRF1 and telomere elongation
Hiroyuki Seimiya et al. Mol Cell Biol. 2004 Mar.
Abstract
In human cells, telomere elongation by telomerase is repressed in cis by the telomeric protein TRF1. Tankyrase 1 binds TRF1 via its ankyrin domain and poly(ADP-ribosyl)ates it. Overexpression of tankyrase 1 in telomerase-positive cells releases TRF1 from telomeres, resulting in telomere elongation. The tankyrase 1 ankyrin domain is classified into five conserved subdomains, ARCs (ankyrin repeat clusters) I to V. Here, we investigated the biological significance of the ARCs. First, each ARC worked as an independent binding site for TRF1. Second, ARCs II to V recognized the N-terminal acidic domain of TRF1 whereas ARC I bound a discrete site between the homodimerization and the Myb-like domains of TRF1. Inactivation of TRF1 binding in the C-terminal ARC, ARC V, either by deletion or point mutation, significantly reduced the ability of tankyrase 1 to poly(ADP-ribosyl)ate TRF1, release TRF1 from telomeres, and elongate telomeres. In contrast, other ARCs, ARC II and/or IV, inactivated by point mutations still retained the biological function of tankyrase 1. On the other hand, ARC V per se was not sufficient for telomere elongation, suggesting a structural role for multiple ARCs. This work provides evidence that specific ARC-TRF1 interactions play roles in the essential catalytic function of tankyrase 1.
Figures
FIG. 1.
Schematic view of tankyrase 1 constructs used in the experiments. (A) LexA-ARC constructs used as described for Fig. 2, 3, and 5. (B and C) FN-tankyrase 1 constructs used as described for Fig. 2D, 4, and 6 to 8. These constructs contain a FLAG epitope tag and an NLS at the N terminus (data not shown). The numbers indicate the positions of amino acid residues. HPS, region containing homopolymeric runs of His, Pro, and Ser; ANK, ankyrin domain; SAM, multimerization domain homologous to the sterile alpha motif; PARP, PARP catalytic domain; ARC, ANK repeat cluster. Bridges above two adjacent ANK repeats indicate the presence of a conserved histidine, contributing to interrepeat stabilization.
FIG. 2.
Tankyrase 1 ARCs work as independent ligand binding sites. (A) In vitro pull-down assay of LexA-ARCs with GST-tankyrase 1 binding proteins. The indicated GST fusions (bound to beads) were incubated with in vitro-translated LexA-ARCs. The bead-bound LexA fusions were detected by Western blot (WB) analysis (left panel). GST fusions were visualized by Coomassie blue staining (right panel). Molecular mass markers (in kilodaltons) are indicated at the right. (B) Phylogenetic tree of ARCs in tankyrase 1 and 2. Amino acid sequences for ARCs were aligned with ClustalW software (
http://www.ddbj.nig.ac.jp/E-mail/clustalw-e.html
). The tree was created using DendroMaker software (
http://www.cib.nig.ac.jp/dda/timanish/dendromaker/home.html
). (C) Interaction of tankyrase 1 with TAB182 is competitively inhibited by TRF1. In vitro-translated FN-tankyrase 1 and TAB182C were incubated with increasing amounts of GST-TRF1 (0, 0.1, or 1 μg) or GST (5 μg). FN-tankyrase 1 was immunoprecipitated (IP) with anti-FLAG antibody, and bead-bound TAB182C was detected by Western blot analysis. The filter was reprobed with anti-GST antibody. (D) The presence of a single functional ARC is sufficient for the interaction of tankyrase 1 with TRF1. Deletion mutants were prepared by in vitro translation and subjected to a pull-down assay with GST-TRF1. The bead-bound proteins were detected by Western blot analysis (left panel). The results of Coomassie staining of GST-TRF1 are also shown (right panel). Molecular mass markers (in kilodaltons) are indicated at the left of each panel.
FIG. 3.
Tankyrase 1 ARCs recognize discrete domains of TRF1. (A) GST-TRF1 constructs used in the experiments. The numbers indicate the positions of amino acid residues. (B) Pull-down assays were performed with in vitro-translated FN-tankyrase 1 and various GST-TRF1 fusions. Bead-bound FN-tankyrase 1 was detected by Western blot (WB) analysis (left panel). GST fusions were visualized by Coomassie blue staining (right panel). Molecular mass markers (in kilodaltons) are indicated to the right of each panel. (C) Specificity of ARCs for TRF1 binding. Pull-down assays were performed with in vitro-translated LexA-ARCs and recombinant GST-TRF1 fusions. (Top panel) The bead-bound LexA-fusions were detected as described for Fig. 2A. (Bottom panel) Deduced ARC binding sites in TRF1. (D) ARCs bind TRF1 in intact cells. LexA-ARCs and Myc-TRF1 or Myc-TRF1(2-210) were transiently expressed in HeLa I.2.11 cells, and the lysates were immunoprecipitated (IP) as indicated. The bead-bound LexA fusions were detected by Western blot analysis.
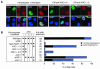
FIG. 4.
Tankyrase 1 deletion mutants retaining ARC V still show TRF1-releasing activity. (A) Effects of transient overexpression of FN-tankyrase 1 constructs on telomeric localization of TRF1 in HeLa I.2.11 cells. Cells were transfected with vectors as indicated. FN-tankyrase 1 constructs, TRF1, and TRF2 were detected by indirect immunofluorescence staining with anti-FLAG M2 (green), anti-TRF1 5747 (red), and anti-TRF2 4A794 (red) antibodies, respectively. DAPI staining of DNA is shown in blue. (B) Quantitation of the TRF1-releasing activity. Cells displaying overexpression of the FN-tankyrase 1 constructs were classified into three categories (depending on the appearance of TRF1 dots): complete disappearance, partial disappearance (i.e., observed but obviously decayed compared with those in surrounding nontransduced cells), and no effect (i.e., intensity comparable with that of mock- and nontransduced cells). Closed circles indicate the existence of intact ARCs.
FIG. 5.
ARC point mutations that disrupt the TRF1 binding. (A) (Top panel) Secondary structure of ANK repeat. Arrows and rectangles indicate the approximate positions of the β-strands and α-helices, respectively. (Middle panel) Alignment of the third ANK repeat (AR) in several ANK family proteins and tankyrase 1 ARCs II, IV, and V. Conserved residues are in boldface type. (Bottom panel) Topological diagram of the secondary-structure elements of typical ARC. Circles indicate the α-helices with helix axes perpendicular to the plane of the figure. The asterisk indicates the conserved proline, which was replaced with leucine in this study. (B) Replacement of the conserved proline with leucine abolished the TRF1 binding of ARCs. In vitro-translated LexA-ARC mutants (mt) were subjected to a pull-down assay with GST-TRF1 or GST (as described for Fig. 2A). The bead-bound LexA fusions were detected by Western blot analysis (WB) (top panels). The results of Coomassie blue staining of GST-TRF1 are also shown (bottom panels).
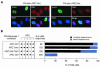
FIG. 6.
Inactivation of ARC V abolishes the TRF1-releasing activity of tankyrase 1. (A) Effects of transient overexpression of the point mutant FN-tankyrase 1 on telomeric localization of TRF1 in HeLa I.2.11 cells. Cells were transfected with vectors as indicated. FN-tankyrase 1 constructs (green), TRF1 (red), and DNA (blue) were detected as described for Fig. 4A. (B) Quantitation of the TRF1-releasing activity. Cells displaying overexpression of the FN-tankyrase 1 constructs were classified into three categories as described for Fig. 4B. Closed and open circles indicate wild-type and Pro→Leu mutant (mt) ARCs, respectively.
FIG. 7.
Differential requirements of multiple ARCs for telomere elongation by tankyrase 1. (A) Effects of overexpression of FN-tankyrase 1 constructs on telomere length in HTC75 cells. TRF at the indicated PD were detected by Southern blot analysis. The results of two representative experiments are shown. M, mock; WT-Tank, wild-type FN-tankyrase 1; mt, mutant. (B) Graphic representations of telomere length change. The plots represent the mean TRF values derived the results presented in panel A. (C) Western blot analysis of whole-cell or nuclear extracts (20 μg of proteins per lane). Blots were probed with antibodies as indicated. For detection of self-poly(ADP-ribosyl)ation, lysates were immunoprecipitated (IP) with anti-FLAG antibody and the pellets were subjected to Western blot analysis with anti-poly(ADP-ribose). MW, molecular mass.
FIG. 8.
Crucial requirement of ARC V for poly(ADP-ribosyl)ation of TRF1 in vitro. (A) Poly(ADP-ribosyl)ation of TRF1 by FN-tankyrase 1 constructs in vitro. Whole-cell extracts were immunoprecipitated with anti-FLAG M2 agarose. The beads were washed and incubated with 4 μg of GST-TRF1 and 1.3 μM [32P]NAD+. +3AB, 1 mM 3-aminobenzamide (3AB) was added prior to addition of GST-TRF1. Reactions were terminated by adding TCA and fractionated by SDS-PAGE. Poly(ADP-ribosyl)ated GST-TRF1 was detected by autoradiography (upper panels). Small aliquots of the beads were subjected to Western blot analysis (WB) with anti-tankyrase antibody (lower panels). mt, mutant. (B) Quantitation of PARP activity in each construct. Radioactivity in the area corresponding to the size of GST-TRF1 was quantitated with a Fuji BAS imaging analyzer. Bars indicate the averages of values obtained in four to five independent experiments. Asterisks indicate statistically significant differences (evaluated using an unpaired t test and FN-tank-ΔANK).
Similar articles
- Telomere elongation by a mutant tankyrase 1 without TRF1 poly(ADP-ribosyl)ation.
Muramatsu Y, Tahara H, Ono T, Tsuruo T, Seimiya H. Muramatsu Y, et al. Exp Cell Res. 2008 Mar 10;314(5):1115-24. doi: 10.1016/j.yexcr.2007.12.005. Epub 2007 Dec 14. Exp Cell Res. 2008. PMID: 18221737 - Role for the related poly(ADP-Ribose) polymerases tankyrase 1 and 2 at human telomeres.
Cook BD, Dynek JN, Chang W, Shostak G, Smith S. Cook BD, et al. Mol Cell Biol. 2002 Jan;22(1):332-42. doi: 10.1128/MCB.22.1.332-342.2002. Mol Cell Biol. 2002. PMID: 11739745 Free PMC article. - Tankyrase function at telomeres, spindle poles, and beyond.
Hsiao SJ, Smith S. Hsiao SJ, et al. Biochimie. 2008 Jan;90(1):83-92. doi: 10.1016/j.biochi.2007.07.012. Epub 2007 Jul 24. Biochimie. 2008. PMID: 17825467 Review. - A cellular survival switch: poly(ADP-ribosyl)ation stimulates DNA repair and silences transcription.
Ziegler M, Oei SL. Ziegler M, et al. Bioessays. 2001 Jun;23(6):543-8. doi: 10.1002/bies.1074. Bioessays. 2001. PMID: 11385634 Review.
Cited by
- A FRET-based high-throughput screening platform for the discovery of chemical probes targeting the scaffolding functions of human tankyrases.
Sowa ST, Vela-Rodríguez C, Galera-Prat A, Cázares-Olivera M, Prunskaite-Hyyryläinen R, Ignatev A, Lehtiö L. Sowa ST, et al. Sci Rep. 2020 Jul 23;10(1):12357. doi: 10.1038/s41598-020-69229-y. Sci Rep. 2020. PMID: 32704068 Free PMC article. - The telomeric PARP, tankyrases, as targets for cancer therapy.
Seimiya H. Seimiya H. Br J Cancer. 2006 Feb 13;94(3):341-5. doi: 10.1038/sj.bjc.6602951. Br J Cancer. 2006. PMID: 16421589 Free PMC article. Review. - Engineering mono- and multi-valent inhibitors on a modular scaffold.
Diamante A, Chaturbedy PK, Rowling PJE, Kumita JR, Eapen RS, McLaughlin SH, de la Roche M, Perez-Riba A, Itzhaki LS. Diamante A, et al. Chem Sci. 2020 Dec 17;12(3):880-895. doi: 10.1039/d0sc03175e. eCollection 2021 Jan 21. Chem Sci. 2020. PMID: 33623657 Free PMC article. - Tankyrase Requires SAM Domain-Dependent Polymerization to Support Wnt-β-Catenin Signaling.
Mariotti L, Templeton CM, Ranes M, Paracuellos P, Cronin N, Beuron F, Morris E, Guettler S. Mariotti L, et al. Mol Cell. 2016 Aug 4;63(3):498-513. doi: 10.1016/j.molcel.2016.06.019. Mol Cell. 2016. PMID: 27494558 Free PMC article. - PARP1 allows proper telomere replication through TRF1 poly (ADP-ribosyl)ation and helicase recruitment.
Maresca C, Dello Stritto A, D'Angelo C, Petti E, Rizzo A, Vertecchi E, Berardinelli F, Bonanni L, Sgura A, Antoccia A, Graziani G, Biroccio A, Salvati E. Maresca C, et al. Commun Biol. 2023 Mar 2;6(1):234. doi: 10.1038/s42003-023-04596-6. Commun Biol. 2023. PMID: 36864251 Free PMC article.
References
- Blackburn, E. H. 2001. Switching and signaling at the telomere. Cell 106:661-673. - PubMed
- Blasco, M. A., H.-W. Lee, M. P. Hande, E. Samper, P. M. Lansdorp, R. A. DePinho, and C. W. Greider. 1997. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91:25-34. - PubMed
- Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C.-P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials