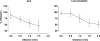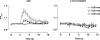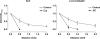Spreading dilatation in rat mesenteric arteries associated with calcium-independent endothelial cell hyperpolarization - PubMed (original) (raw)
Spreading dilatation in rat mesenteric arteries associated with calcium-independent endothelial cell hyperpolarization
Hiromichi Takano et al. J Physiol. 2004.
Abstract
Both ACh and levcromakalim evoke smooth muscle cell hyperpolarization and associated relaxation in rat mesenteric resistance arteries. We investigated if they could evoke conducted vasodilatation along isolated arteries, whether this reflected spreading hyperpolarization and the possible mechanism involved. Focal micropipette application of either ACh, to stimulate endothelial cell muscarinic receptors, or levcromakalim, to activate smooth muscle K(ATP) channels, each evoked a local dilatation (88 +/- 14%, n= 6 and 92 +/- 6% reversal of phenylephrine-induced tone, n= 11, respectively) that rapidly spread upstream (at 1.5 mm 46 +/- 19%, n= 6 and 57 +/- 13%, n= 9) to dilate the entire isolated artery. The local dilatation to ACh was associated with a rise in endothelial cell [Ca(2+)](i) (F/F(t = 0)= 1.22 +/- 0.33, n= 14) which did not spread beyond 0.5 mm (F/F(t = 0)= 1.01 +/- 0.01, n= 14), while the local dilatation to levcromakalim was not associated with any change in endothelial cell [Ca(2+)](i). In contrast, ACh and levcromakalim both stimulated local (12.7 +/- 1.2 mV, n= 10 and 13.5 +/- 4.7 mV, n= 10) and spreading (at 2 mm: 3.0 +/- 1.1 mV, n= 5 and 4.1 +/- 0.7 mV, n= 5) smooth muscle hyperpolarization. The spread of hyperpolarization could be prevented by cutting the artery, so was not due to a diffusible agent. Both the spreading dilatation and hyperpolarization were endothelium dependent. The injection of propidium iodide into either endothelial or smooth muscle cells revealed extensive dye coupling between the endothelial cells, but limited coupling between the smooth muscle cells. Some evidence for heterocellular spread of dye was also evident. Together, these data show that vasodilatation can spread over significant distances in mesenteric resistance arteries, and suggest this reflects an effective coupling between the endothelial cells to facilitate [Ca(2+)](i)-independent spread of hyperpolarization.
Figures
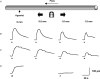
Figure 1. Representative records of local and conducted dilatation in response to agonists
A, schematic diagram of the experimental paradigm in pressurized arteries. Each agonist was pressure-pulse ejected at the local site (0 mm) and the microscope moved independently of the artery and pipette (which remained fixed). This enabled observation of diameter along the artery length whilst maintaining a constant position of stimulation. The superfusion flow prevented the passive diffusion of agonist to the upstream sites. B–D, time course of changes in artery diameter in response to local application of agonist at the time indicated by each triangle. The artery dilated rapidly upon focal application of ACh (B, 10−3
m
, 30 ms) and levcromakalim (C, 10−3
m
, 30 ms) at all observation sites along the artery length. For each observation, the artery wall dilated synchronously, with decay in amplitude with distance. D, in endothelium-denuded arteries, the duration of levcromakalim stimulation was increased to 300 ms, to evoke local responses of a similar magnitude to responses in endothelium-intact arteries. In these experiments, the dilatation only occurred near the site of stimulation, and by 1 mm upstream no change in diameter was observed.
Figure 2. Local and conducted dilatation evoked by ACh and levcromakalim
Summarized data showing the average peak increase in diameter (% dilatation) along the artery length stimulated by ACh (left panel, _n_= 6) and levcromakalim (right panel, _n_= 6–11). Both agonists were pressure-pulse ejected (10−3
m
for 30 ms) at the local site (0 mm) and changes in diameter were observed at sites upstream and against the direction of superfusion flow. All arteries were precontracted with phenylephrine (1−3 × 10−7
m
) to stimulate approximately 20% tone.
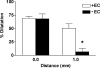
Figure 3. The endothelium facilitated conducted dilatation in response to levcromakalim
Summarized data showing the average peak increase in diameter (% dilatation) stimulated by levcromaklim in endothelium-intact (+EC, _n_= 3) and endothelium-denuded (–EC, _n_= 3) arteries. The duration of focal (at 0 mm) pressure-pulse ejection was titrated to obtain similar magnitudes of local dilatation. At 1 mm upstream from the stimulation pipette the amplitude of dilatation was markedly reduced in the denuded artery, supporting the crucial importance of the endothelium in facilitating the spread of dilatation. All arteries were precontracted with phenylephrine (1−3 × 10−7
m
) to stimulate approximately 20% tone. *P < 0.05 versus+EC at 1 mm.
Figure 4. Representative images of local and conducted changes in endothelial cell [Ca2+]i in response to ACh
Pressurized arteries were luminally loaded with the fluorescent Ca2+ indicator fluo-4 selectively to load endothelial cells. A, fluorescence image of the lower wall of the artery at the plane of the endothelial cells, equivalent to _F_t = 0. The position of the stimulation pipette is visible at the bottom of the image (also indicated by arrow in E) and the flow of superfusate are is from left to right to avoid upstream diffusion of ACh. B–E, sequential F/_F_t = 0 images of the same artery, where ACh was pressure-pulse ejected (10−3
m
, 300 ms) at _t_= 0 s, and F acquired at the times indicated in the top left of each image. Note that in B the rise in endothelial cell [Ca2+]i occurred in the vicinity of the pipette, and subsequently (C–E) spread both downstream (longitudinally) and across (radially) the artery wall, but not to regions beyond 400 μm upstream of the pipette. Addition of ACh (10−6
m
) to the bath increased endothelial cell fluorescence intensity across the entire length of artery (not shown). Bar = 100 μm.
Figure 5. Local and conducted changes in endothelial cell [Ca2+]i in response to ACh and levcromakalim
Summarized data showing the average time course of changes in fluorescence intensity (F/_F_t = 0) stimulated by ACh (left panel, _n_= 14) and levcromakalim (right panel, _n_= 15) along the artery length. Both agonists were pressure-pulse ejected (10−3
m
, 300 or 100 ms, respectively) at the local site (0 mm) and changes in fluorescence observed at sites upstream and against the direction of superfusion flow. Note that a rise in endothelial cell [Ca2+]i only occurred in response to ACh, and only in regions less than 500 μm upstream of the stimulation pipette. Phenylephrine was not present in these experiments.
Figure 6. Representative records of local and conducted hyperpolarization to agonists
A, schematic diagram of the experimental paradigm in pinned-out arteries. A smooth muscle cell was impaled with a glass microelectrode at the local site (0 mm) and the position of the stimulation pipette moved independently of the artery and microelectrode (which remained fixed). This enabled measurement of membrane potential at increasing distance from the stimulation pipette during a single impalement. The superfusion flow prevented the passive diffusion of agonist to the upstream sites. B–D, time course of changes in membrane potential in response to local application of agonist at the time indicated by each triangle. The artery hyperpolarized rapidly upon focal application of ACh (B, 10−3
m
, 5 ms) and levcromakalim (C, 10−3
m
, 20 ms) at all observation sites along the artery length, with decay in amplitude with distance. D, in endothelium-denuded arteries, the duration of levcromakalim stimulation was increased to 30 ms, to evoke local responses of a similar magnitude to those in endothelium-intact arteries. In these experiments, the magnitude of hyperpolarization relative to the local response (AUC/AUClocal) decreased markedly with distance compared to the endothelium-intact arteries. Note that the time scale for A is different to B and C.
Figure 7. Local and conducted hyperpolarization evoked by ACh and levcromakalim
Summarized data showing the average increase in membrane potential with time (AUC, mV s) along the artery length relative to the local AUC (AUC/AUClocal). Both agonists were pressure-pulse ejected at positions along the artery (0–2 mm from microelectrode) and membrane potential recorded at the local site (0 mm). The spread of hyperpolarization in response to ACh (10−3
m
, 5–100 ms) observed under control conditions (left panel, _n_= 4–9) was abolished by cutting the artery 200 μm downstream from the recording electrode (cut, _n_= 2). The spread of hyperpolarization in response to levcromakalim (10−3
m
, 10–30 ms) under control conditions (right panel, _n_= 4–10) was markedly reduced in endothelium-denuded arteries (– EC, _n_= 3). Phenylephrine was not present in these experiments.
Figure 8. Representative records of endothelial cell hyperpolarization to agonists
Time course of membrane potential change in response to either ACh or levcromakalim in the superfusate. A and B, intracellular microelectrode records of endothelial cell membrane potential from intact arteries. In A, both ACh (10−5
m
) and levcromakalim (10−6
m
) stimulated hyperpolarization. The subsequent addition of glibenclamide (10−5
m
) fully reversed the levcromakalim response. The resting membrane potential in this cell was −54.0 mV. In a separate experiment (B), prior application of glibenclamide blocked the hyperpolarization to levcromakalim, but not to ACh. The resting membrane potential was −53.7 mV. On average, membrane potential increased from −50.4 ± 1.1 mV to −68.8 ± 2.2 mV with ACh, and from −51.0 ± 1.3 to −72.4 ± 3.1 mV with levcromakalim (_n_= 5). C, measurement of membrane potential in a freshly isolated endothelial cell with a patch-electrode. In contrast to intact arteries, levcromakalim did not evoke hyperpolarization. The resting membrane potential of the isolated endothelial cells was −13.6 mV, which was increased to −32.4 mV by current injection (at the point indicated by the arrow). ACh (3 × 10−6
m
) hyperpolarized these cells to −66.1 and −66.6 mV (first and second additions), whereas in the same cell levcromakalim (10−5
m
) had no effect. The period of agonist and antagonist addition is indicated by bars.
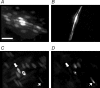
Figure 9. Assessment of homocellular and heterocellular coupling by dye injection
A single endothelial cell (A,C) or smooth muscle cell (B) was impaled with a glass microelectrode containing propidium iodide (1%). Dye appeared to pass more readily to adjacent endothelial cells than to adjacent smooth muscle cells, and on two occasions (from 6) passed from an endothelial cell to underlying smooth muscle cell. C and D, images collected showing propidium iodide staining (C), and then SYTOX Green staining of the same region (D). The injected cell is marked by the asterisk, where the resting membrane potential was −52 mV. The thick (upper) arrows point to a cell where propidium iodide fluorescence is evident in a cell adjacent to the injected cell (C), but no SYTOX Green fluorescence was observed in the same cell (D, suggesting dye-coupling), whereas the thin (lower) arrows show SYTOX Green staining a damaged cell (D) which has very low intensity in C. Bar = 50 μm.
Similar articles
- Endothelial cell Ca2+ increases are independent of membrane potential in pressurized rat mesenteric arteries.
McSherry IN, Spitaler MM, Takano H, Dora KA. McSherry IN, et al. Cell Calcium. 2005 Jul;38(1):23-33. doi: 10.1016/j.ceca.2005.03.007. Cell Calcium. 2005. PMID: 15907999 - Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries.
Dora KA, Gallagher NT, McNeish A, Garland CJ. Dora KA, et al. Circ Res. 2008 May 23;102(10):1247-55. doi: 10.1161/CIRCRESAHA.108.172379. Epub 2008 Apr 10. Circ Res. 2008. PMID: 18403729 Free PMC article. - NO and KATP channels underlie endotoxin-induced smooth muscle hyperpolarization in rat mesenteric resistance arteries.
Wu CC, Chen SJ, Garland CJ. Wu CC, et al. Br J Pharmacol. 2004 Jun;142(3):479-84. doi: 10.1038/sj.bjp.0705794. Epub 2004 May 17. Br J Pharmacol. 2004. PMID: 15148259 Free PMC article. - Spreading vasodilatation in resistance arteries.
Takano H, Dora KA, Garland CJ. Takano H, et al. J Smooth Muscle Res. 2005 Dec;41(6):303-11. doi: 10.1540/jsmr.41.303. J Smooth Muscle Res. 2005. PMID: 16557004 Review. - What is the mechanism of flow-mediated arterial dilatation.
Markos F, Ruane O'Hora T, Noble MI. Markos F, et al. Clin Exp Pharmacol Physiol. 2013 Aug;40(8):489-94. doi: 10.1111/1440-1681.12120. Clin Exp Pharmacol Physiol. 2013. PMID: 23692253 Review.
Cited by
- A mathematical model of vasoreactivity in rat mesenteric arterioles. II. Conducted vasoreactivity.
Kapela A, Nagaraja S, Tsoukias NM. Kapela A, et al. Am J Physiol Heart Circ Physiol. 2010 Jan;298(1):H52-65. doi: 10.1152/ajpheart.00546.2009. Epub 2009 Oct 23. Am J Physiol Heart Circ Physiol. 2010. PMID: 19855062 Free PMC article. - Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries.
Winter P, Dora KA. Winter P, et al. J Physiol. 2007 Jul 1;582(Pt 1):335-47. doi: 10.1113/jphysiol.2007.135202. Epub 2007 May 3. J Physiol. 2007. PMID: 17478526 Free PMC article. - Hydrogen peroxide as an endothelium-derived hyperpolarizing factor.
Shimokawa H. Shimokawa H. Pflugers Arch. 2010 May;459(6):915-22. doi: 10.1007/s00424-010-0790-8. Epub 2010 Feb 6. Pflugers Arch. 2010. PMID: 20140449 Review. - Membrane hyperpolarization is not required for sustained muscarinic agonist-induced increases in intracellular Ca2+ in arteriolar endothelial cells.
Cohen KD, Jackson WF. Cohen KD, et al. Microcirculation. 2005 Mar;12(2):169-82. doi: 10.1080/10739680590904973. Microcirculation. 2005. PMID: 15824039 Free PMC article. - Non-linear relationship between hyperpolarisation and relaxation enables long distance propagation of vasodilatation.
Wölfle SE, Chaston DJ, Goto K, Sandow SL, Edwards FR, Hill CE. Wölfle SE, et al. J Physiol. 2011 May 15;589(Pt 10):2607-23. doi: 10.1113/jphysiol.2010.202580. Epub 2011 Mar 21. J Physiol. 2011. PMID: 21486765 Free PMC article.
References
- Beny JL, Connat JL. An electron-microscopic study of smooth muscle cell dye coupling in the pig coronary arteries. Role of gap junctions. Circ Res. 1992;70:49–55. - PubMed
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. - PubMed
- Busse R, Fichtner H, Luckhoff A, Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine- stimulated endothelial cells. Am J Physiol. 1988;255:H965–H969. - PubMed
- Chen GF, Cheung DW. Effect of K+-channel blockers on ACh-induced hyperpolarization and relaxation in mesenteric arteries. Am J Physiol. 1997;272:H2306–H2312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous