Exogenous pathogen and plant 15-lipoxygenase initiate endogenous lipoxin A4 biosynthesis - PubMed (original) (raw)
Exogenous pathogen and plant 15-lipoxygenase initiate endogenous lipoxin A4 biosynthesis
Gerard L Bannenberg et al. J Exp Med. 2004.
Abstract
Lipoxin A4 (LXA4) is a potent endogenous lipoxygenase-derived eicosanoid with antiinflammatory and proresolving properties. Supraphysiological levels of LXA4 are generated during infection by Toxoplasma gondii, which in turn reduces interleukin (IL) 12 production by dendritic cells, thus dampening Th1-type cell-mediated immune responses and host immunopathology. In the present work, we sought evidence for the structural basis of T. gondii's ability to activate LXA4 biosynthesis. Proteomic analysis of T. gondii extract (soluble tachyzoite antigen [STAg]), which preserves the immunosuppressive and antiinflammatory activity of the parasite, yielded several peptide matches to known plant lipoxygenases. Hence, we incubated STAg itself with arachidonic acid and found using LC-UV-MS-MS-based lipidomics that STAg produced both 15-HETE and 5,15-diHETE, indicating that T. gondii carries 15-lipoxygenase activity. In addition, T. gondii tachyzoites (the rapidly multiplying and invasive stage of the parasite) generated LXA4 when provided with arachidonic acid. Local administration of a plant (soybean) lipoxygenase itself reduced neutrophilic infiltration in murine peritonitis, demonstrating that 15-lipoxygenase possesses antiinflammatory properties. Administration of plant 15-lipoxygenase generated endogenous LXA4 and mimicked the suppression of IL-12 production by splenic dendritic cells observed after T. gondii infection or STAg administration. Together, these results indicate that 15-lipoxygenase expressed by a pathogen as well as exogenously administered 15-lipoxygenase can interact with host biosynthetic circuits for endogenous "stop signals" that divert the host immune response and limit acute inflammation.
Figures
Figure 1.
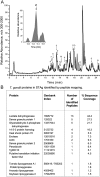
LC-MS-MS analysis of tryptic peptides from T. gondii protein extract (STAg). (A) Arrows indicate the position of the following protein peptide fragments: A, lactate dehydrogenase; B, enolase; C, dense granule protein 1; D, heat shock protein 70; E, dense granule protein 2; L1, peptide match to Lycopersicon esculentum lipoxygenase A; and L2, peptide match to Persea americana lipoxygenase. (inset) Base peak ion trace for peptide L1. (B) T. gondii proteins identified in STAg.
Figure 2.
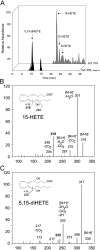
Lipidomic analysis. Profile of lipoxygenase products formed by STAg extracts. (A) Selected ion chromatogram for 15-HETE, 12-HETE, 5-HETE, and 5,15-diHETE formed by 15 μg STAg incubated with 30 μg arachidonic acid in 0.5 ml DPBS, pH 7.4, for 30 min (see Materials and Methods). Tandem mass spectra for the lipoxygenase products 15-HETE (B) and 5,15-diHETE (C). Results are representative of three independent incubations.
Figure 3.
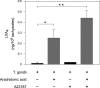
LXA4 formation by T. gondii tachyzoites. T. gondii tachyzoites (107 organisms/well) were incubated for 30 min in the presence or absence of 5 μM A23187 and 20 μM arachidonic acid. LXA4 was monitored (see Materials and Methods) and expressed as mean values ± SEM (n = 3). Student's t test; *, P < 0.05; **, P < 0.005.
Figure 4.
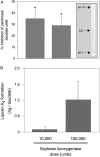
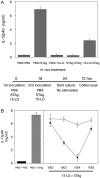
Local administration of plant 15-lipoxygenase diminishes zymosan A–stimulated leukocyte infiltration, a hallmark of acute inflammation. Peritonitis was induced by intraperitoneal injection of 1 mg zymosan A. Soybean lipoxygenase (10 or 100 × 103 U) or vehicle alone was administered 5 min before zymosan A (1 mg/ml saline i.p.), and after 2 h, peritoneal exudates were collected. (A) Inhibition of peritoneal exudate cell numbers (mean ± SEM; n = 5–7; *, significantly different from vehicle; P < 0.05). (inset) In-gel lipoxygenase activity stain with substrate arachidonic acid after native isoelectric focusing of soybean 15-lipoxygenase (LO; 5 × 103 U). (B) Peritoneal exudate LXA4 levels expressed as mean values ± SEM (n = 7–8).
Figure 5.
Plant 15-lipoxygenase reduces dendritic cell IL-12 production in vivo. (A) Intraperitoneal administration of plant 15-lipoxygenase mimics STAg-induced paralysis of splenic dendritic cells. Mice were injected i.p. with 1 μg STAg, 103 U soybean lipoxygenase, or vehicle alone 18 h before a second challenge with 1 μg STAg, 103 U soybean lipoxygenase, or vehicle. 6 h later, splenic dendritic cells were harvested and cultured for another 12 h. Dendritic cell formation of IL-12 p40 was measured (see Materials and Methods) and expressed as mean values ± SEM (n = 3). (B) Dose-dependent induction of dendritic cell paralysis (see Introduction and references – and for details) by i.p. administration of plant 15-lipoxygenase requires catalytic activity. Mice were injected intraperitoneally with native (closed circles), boiled (open circles) soybean lipoxygenase, or vehicle alone 18 h before a second challenge with 1 μg STAg (i.p.) or vehicle. 6 h later, splenic dendritic cells were harvested and cultured for another 12 h. IL-12 p40 was measured (see Materials and Methods) and expressed as mean values ± SEM (n = 3).
Figure 6.
Hypothetical scheme for bidirectional microbial and plant 15-lipoxygenase–initiated biosynthetic pathways that enhance endogenous LXA4 formation.
Similar articles
- Lipoxin A4 and lipoxin B4 stimulate the release but not the oxygenation of arachidonic acid in human neutrophils: dissociation between lipid remodeling and adhesion.
Nigam S, Fiore S, Luscinskas FW, Serhan CN. Nigam S, et al. J Cell Physiol. 1990 Jun;143(3):512-23. doi: 10.1002/jcp.1041430316. J Cell Physiol. 1990. PMID: 2162850 - Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection.
Aliberti J, Serhan C, Sher A. Aliberti J, et al. J Exp Med. 2002 Nov 4;196(9):1253-62. doi: 10.1084/jem.20021183. J Exp Med. 2002. PMID: 12417634 Free PMC article. - Human alveolar macrophages have 15-lipoxygenase and generate 15(S)-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid and lipoxins.
Levy BD, Romano M, Chapman HA, Reilly JJ, Drazen J, Serhan CN. Levy BD, et al. J Clin Invest. 1993 Sep;92(3):1572-9. doi: 10.1172/JCI116738. J Clin Invest. 1993. PMID: 8376607 Free PMC article. - Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution.
Serhan CN. Serhan CN. Prostaglandins Leukot Essent Fatty Acids. 2005 Sep-Oct;73(3-4):141-62. doi: 10.1016/j.plefa.2005.05.002. Prostaglandins Leukot Essent Fatty Acids. 2005. PMID: 16005201 Review. - Positive and negative regulation of pathogen induced dendritic cell function by G-protein coupled receptors.
Aliberti J, Sher A. Aliberti J, et al. Mol Immunol. 2002 May;38(12-13):891-3. doi: 10.1016/s0161-5890(02)00015-9. Mol Immunol. 2002. PMID: 12009566 Review.
Cited by
- Pro-Resolving Ligands Orchestrate Phagocytosis.
Decker C, Sadhu S, Fredman G. Decker C, et al. Front Immunol. 2021 Jun 10;12:660865. doi: 10.3389/fimmu.2021.660865. eCollection 2021. Front Immunol. 2021. PMID: 34177900 Free PMC article. Review. - Co-opting oxylipin signals in microbial disease.
Niu M, Keller NP. Niu M, et al. Cell Microbiol. 2019 Jun;21(6):e13025. doi: 10.1111/cmi.13025. Cell Microbiol. 2019. PMID: 30866138 Free PMC article. Review. - TB: the Yin and Yang of lipid mediators.
Tobin DM, Ramakrishnan L. Tobin DM, et al. Curr Opin Pharmacol. 2013 Aug;13(4):641-5. doi: 10.1016/j.coph.2013.06.007. Epub 2013 Jul 9. Curr Opin Pharmacol. 2013. PMID: 23849093 Free PMC article. Review. - Lipoxins: resolutionary road.
Maderna P, Godson C. Maderna P, et al. Br J Pharmacol. 2009 Oct;158(4):947-59. doi: 10.1111/j.1476-5381.2009.00386.x. Epub 2009 Sep 28. Br J Pharmacol. 2009. PMID: 19785661 Free PMC article. Review. - Transcriptomic analyses of murine resolution-phase macrophages.
Stables MJ, Shah S, Camon EB, Lovering RC, Newson J, Bystrom J, Farrow S, Gilroy DW. Stables MJ, et al. Blood. 2011 Dec 22;118(26):e192-208. doi: 10.1182/blood-2011-04-345330. Epub 2011 Oct 19. Blood. 2011. PMID: 22012065 Free PMC article.
References
- Samuelsson, B., S.E. Dahlén, J.A. Lindgren, C.A. Rouzer, and C.N. Serhan. 1987. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 237:1171–1176. - PubMed
- Serhan, C.N. 1994. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim. Biophys. Acta. 1212:1–25. - PubMed
- Nathan, C. 2002. Points of control in inflammation. Nature. 420:846–852. - PubMed
- Bandeira-Melo, C., P.T. Bozza, B.L. Diaz, R.S. Cordeiro, P.J. Jose, M.A. Martins, and C.N. Serhan. 2000. Cutting edge: lipoxin (LX) A4 and aspirin-triggered 15-epi-LXA4 block allergen-induced eosinophil trafficking. J. Immunol. 164:2267–2271. - PubMed
- Ramstedt, U., J. Ng, H. Wigzell, C.N. Serhan, and B. Samuelsson. 1985. Action of novel eicosanoids lipoxin A and B on human natural killer cell cytotoxicity: effects on intracellular cAMP and target cell binding. J. Immunol. 135:3434–3438. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM038765/GM/NIGMS NIH HHS/United States
- P01-DE13499/DE/NIDCR NIH HHS/United States
- P01 DK050305/DK/NIDDK NIH HHS/United States
- P01-DK50305/DK/NIDDK NIH HHS/United States
- P01 DE013499/DE/NIDCR NIH HHS/United States
- R37 GM038765/GM/NIGMS NIH HHS/United States
- GM-38765/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources