Digital karyotyping identifies thymidylate synthase amplification as a mechanism of resistance to 5-fluorouracil in metastatic colorectal cancer patients - PubMed (original) (raw)
. 2004 Mar 2;101(9):3089-94.
doi: 10.1073/pnas.0308716101. Epub 2004 Feb 17.
Luis A Diaz Jr, Katharine Romans, Alberto Bardelli, Saurabh Saha, Gennaro Galizia, Michael Choti, Ross Donehower, Giovanni Parmigiani, Ie-Ming Shih, Christine Iacobuzio-Donahue, Kenneth W Kinzler, Bert Vogelstein, Christoph Lengauer, Victor E Velculescu
Affiliations
- PMID: 14970324
- PMCID: PMC420348
- DOI: 10.1073/pnas.0308716101
Digital karyotyping identifies thymidylate synthase amplification as a mechanism of resistance to 5-fluorouracil in metastatic colorectal cancer patients
Tian-Li Wang et al. Proc Natl Acad Sci U S A. 2004.
Abstract
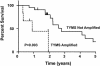
Resistance to chemotherapy is a major cause of mortality in advanced cancer patients. In this study, digital karyotyping was used to search for genomic alterations in liver metastases that were clinically resistant to 5-fluorouracil (5-FU). In two of four patients, we identified amplification of an approximately 100-kb region on 18p11.32 that was of particular interest because it contained the gene encoding thymidylate synthase (TYMS), a molecular target of 5-FU. Analysis of TYMS by fluorescence in situ hybridization identified TYMS gene amplification in 23% of 31 5-FU-treated cancers, whereas no amplification was observed in metastases of patients that had not been treated with 5-FU. Patients with metastases containing TYMS amplification had a substantially shorter median survival (329 days) than those without amplification (1,021 days, P <0.01). These data suggest that genetic amplification of TYMS is a major mechanism of 5-FU resistance in vivo and have important implications for the management of colorectal cancer patients with recurrent disease.
Figures
Fig. 1.
Overlapping regions of amplification on chromosome 18p identified by DK. Bitmap views comprised of 18,431 pixels representing tag density values at the chromosomal position of each virtual tag on chromosome 18. Yellow regions indicate tag densities that were not amplified, and black regions represent areas with genomic tag densities indicating amplification. Genomic tag densities were determined as described in Materials and Methods and had maximal values of 10 and 6 copies per diploid genome for the amplifications in FU-M2 and FU-M4, respectively. Genes present within overlapping amplified regions are indicated below on a high-resolution map. Only TYMS and rTS were entirely contained within the regions that were amplified in both FU-M2 and FU-M4.
Fig. 2.
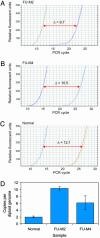
Quantitative PCR analysis of genomic DNA from colorectal metastases. Quantitive PCR analysis of TYMS (right curves) and LINE element control (left curves) performed on genomic DNA from colorectal cancer metastases FU-M2 (A) and FU-M4 (B) and normal (nontumor) DNA (C). (D) Differences in threshold cycle numbers between LINE element and TYMS confirm that TYMS is present at increased gene copy numbers in colorectal metastases.
Fig. 3.
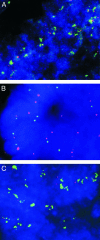
TYMS amplification assessed by interphase FISH. Analysis of interphase nuclei from a colorectal cancer metastasis to the liver after 5-FU treatment (A). Matched colorectal adenoma obtained before 5-FU treatment (B) and colorectal cancer obtained after 5-FU neoadjuvant treatment (C) from a patient with familial adenomatous polyposis. Nuclei are visualized with 4′,6′-diamidino-2-phenylindole stain (blue); TYMS probe (located on chromosome 18p, 0.8-1.0 Mb from the telomere) is visualized by using FITC-avidin (green), and chromosome 18 control probe (located on chromosome 18p, 13.0-13.2 Mb from the telomere) is visualized by using tetramethylrhodamine B isothiocyanate-conjugated antibodies (red). Increased TYMS gene copy number was observed only in patients previously treated with 5-FU (A and C). (Magnification: ×600.)
Fig. 4.
Five-year survival curve for patients with and without TYMS amplification.
Similar articles
- Amplification of thymidylate synthetase in metastatic colorectal cancer patients pretreated with 5-fluorouracil-based chemotherapy.
Watson RG, Muhale F, Thorne LB, Yu J, O'Neil BH, Hoskins JM, Meyers MO, Deal AM, Ibrahim JG, Hudson ML, Walko CM, McLeod HL, Auman JT. Watson RG, et al. Eur J Cancer. 2010 Dec;46(18):3358-64. doi: 10.1016/j.ejca.2010.07.011. Epub 2010 Aug 18. Eur J Cancer. 2010. PMID: 20727737 Free PMC article. - Thymidylate synthase expression in circulating tumor cells: a new tool to predict 5-fluorouracil resistance in metastatic colorectal cancer patients.
Abdallah EA, Fanelli MF, Buim ME, Machado Netto MC, Gasparini Junior JL, Souza E Silva V, Dettino AL, Mingues NB, Romero JV, Ocea LM, Rocha BM, Alves VS, Araújo DV, Chinen LT. Abdallah EA, et al. Int J Cancer. 2015 Sep 15;137(6):1397-405. doi: 10.1002/ijc.29495. Epub 2015 Mar 11. Int J Cancer. 2015. PMID: 25721610 Free PMC article. - Thymidylate synthase gene polymorphism predicts toxicity in colorectal cancer patients receiving 5-fluorouracil-based chemotherapy.
Lecomte T, Ferraz JM, Zinzindohoué F, Loriot MA, Tregouet DA, Landi B, Berger A, Cugnenc PH, Jian R, Beaune P, Laurent-Puig P. Lecomte T, et al. Clin Cancer Res. 2004 Sep 1;10(17):5880-8. doi: 10.1158/1078-0432.CCR-04-0169. Clin Cancer Res. 2004. PMID: 15355920 - Thymidylate synthase pharmacogenetics in colorectal cancer.
Marsh S, McLeod HL. Marsh S, et al. Clin Colorectal Cancer. 2001 Nov;1(3):175-8; discussion 179-81. doi: 10.3816/CCC.2001.n.018. Clin Colorectal Cancer. 2001. PMID: 12450432 Review. - [Response marker to 5-FU after curative surgery in colorectal cancer].
Tsuji T. Tsuji T. Nihon Rinsho. 2003 Sep;61 Suppl 7:325-8. Nihon Rinsho. 2003. PMID: 14574904 Review. Japanese. No abstract available.
Cited by
- Overlooking evolution: a systematic analysis of cancer relapse and therapeutic resistance research.
Aktipis CA, Kwan VS, Johnson KA, Neuberg SL, Maley CC. Aktipis CA, et al. PLoS One. 2011;6(11):e26100. doi: 10.1371/journal.pone.0026100. Epub 2011 Nov 17. PLoS One. 2011. PMID: 22125594 Free PMC article. - Delimiting Allelic Imbalance of TYMS by Allele-Specific Analysis.
Balboa-Beltrán E, Cruz R, Carracedo A, Barros F. Balboa-Beltrán E, et al. Medicine (Baltimore). 2015 Jul;94(27):e1091. doi: 10.1097/MD.0000000000001091. Medicine (Baltimore). 2015. PMID: 26166093 Free PMC article. - Genomic plasticity of the human fungal pathogen Candida albicans.
Selmecki A, Forche A, Berman J. Selmecki A, et al. Eukaryot Cell. 2010 Jul;9(7):991-1008. doi: 10.1128/EC.00060-10. Epub 2010 May 21. Eukaryot Cell. 2010. PMID: 20495058 Free PMC article. Review. - Liquid biopsies: genotyping circulating tumor DNA.
Diaz LA Jr, Bardelli A. Diaz LA Jr, et al. J Clin Oncol. 2014 Feb 20;32(6):579-86. doi: 10.1200/JCO.2012.45.2011. Epub 2014 Jan 21. J Clin Oncol. 2014. PMID: 24449238 Free PMC article. Review. - Glioblastoma proto-oncogene SEC61gamma is required for tumor cell survival and response to endoplasmic reticulum stress.
Lu Z, Zhou L, Killela P, Rasheed AB, Di C, Poe WE, McLendon RE, Bigner DD, Nicchitta C, Yan H. Lu Z, et al. Cancer Res. 2009 Dec 1;69(23):9105-11. doi: 10.1158/0008-5472.CAN-09-2775. Epub 2009 Nov 17. Cancer Res. 2009. PMID: 19920201 Free PMC article.
References
- Moertel, C. G. (1994) N. Engl. J. Med. 330, 1136-1142. - PubMed
- Longley, D. B., Harkin, D. P. & Johnston, P. G. (2003) Nat. Rev. Cancer 3, 330-338. - PubMed
- Giacchetti, S., Perpoint, B., Zidani, R., Le Bail, N., Faggiuolo, R., Focan, C., Chollet, P., Llory, J. F., Letourneau, Y., Coudert, B., et al. (2000) J. Clin. Oncol. 18, 136-147. - PubMed
- de Gramont, A., Figer, A., Seymour, M., Homerin, M., Hmissi, A., Cassidy, J., Boni, C., Cortes-Funes, H., Cervantes, A., Freyer, G., et al. (2000) J. Clin. Oncol. 18, 2938-2947. - PubMed
- Saltz, L. B., Cox, J. V., Blanke, C., Rosen, L. S., Fehrenbacher, L., Moore, M. J., Maroun, J. A., Ackland, S. P., Locker, P. K., Pirotta, N., et al. (2000) N. Engl. J. Med. 343, 905-914. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical