Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing - PubMed (original) (raw)
Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing
Gunter Meister et al. RNA. 2004 Mar.
Abstract
A large number of miRNAs have recently been discovered in plants and animals. Development of reverse genetic approaches that act to inhibit microRNA function would facilitate the study of this new class of noncoding RNA. Here we show that 2'-O-methyl oligoribonucleotides, but not 2'-deoxyoligonucleotides specifically inactivate the RNAi activity associated with miRNA-protein complexes in human cell extracts as well as in cultured human cells.
Figures
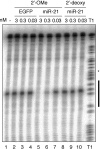
FIGURE 1.
Antisense 2′-O-methyl oligoribonucleotide specifically inhibit miR-21 guided cleavage activity in HeLa cell S100 cytoplasmic extracts. The black bar to the left of the RNase T1 ladder represents the region of the target RNA complementary to miR-21. Oligonucleotides complementary to miR-21 were preincubated in S100 extracts prior to the addition of 32P-cap-labeled cleavage substrate. Cleavage bands and T1 hydrolysis bands appear as doublets after a 1-nt slipping of the T7 RNA polymerase near the middle of the transcript indicated by the asterisk.
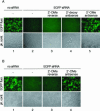
FIGURE 2.
Antisense 2′-O-methyl oligoribonucleotides block EGFP-targeting RISC in EGFP stably expressing HeLa cells. (A) EGFP expressing HeLa cells were cotransfected with EGFP siRNA duplex and the indicated single-stranded oligonucleotides. EGFP fluorescence was recorded 48 h past transfection. The antisense 2′-OMe oligoribonucleotide is complementary to the guide siRNA strand. The reversed sequence (not the reverse complement) of antisense 2′-OMe oligoribonucleotide was used as control. The guide siRNA is complementary to a segment of the EGFP mRNA. The top row shows fluorescence microscopy images recording the EGFP fluorescence; the bottom row shows phase contrast images. (B) EGFP expressing HeLa cells were first transfected with EGFP siRNA duplex followed by transfection of and the indicated single-stranded 2′-OMe oligoribonucleotides after 24 h.
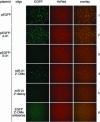
FIGURE 3.
Antisense 2′-O-methyl oligoribonucleotides interfere with endogenous miR-21 RNP cleavage in HeLa cells. HeLa cells were transfected with pHcRed and pEGFP or its derivatives, with or without inhibitory or control oligonucleotides. EGFP and HcRed protein fluorescence were excited and recorded individually by fluorescence microscopy 24 h after transfection. Coexpression of cotransfected reporter plasmids was documented by superimposing of the fluorescence images in the right panel.
Similar articles
- siRNAs can function as miRNAs.
Doench JG, Petersen CP, Sharp PA. Doench JG, et al. Genes Dev. 2003 Feb 15;17(4):438-42. doi: 10.1101/gad.1064703. Genes Dev. 2003. PMID: 12600936 Free PMC article. - [Great potential of small RNAs: RNA interference and microRNA].
Vázquez-Ortiz G, Piña-Sánchez P, Salcedo M. Vázquez-Ortiz G, et al. Rev Invest Clin. 2006 Jul-Aug;58(4):335-49. Rev Invest Clin. 2006. PMID: 17146945 Review. Spanish. - A small molecule enhances RNA interference and promotes microRNA processing.
Shan G, Li Y, Zhang J, Li W, Szulwach KE, Duan R, Faghihi MA, Khalil AM, Lu L, Paroo Z, Chan AW, Shi Z, Liu Q, Wahlestedt C, He C, Jin P. Shan G, et al. Nat Biotechnol. 2008 Aug;26(8):933-40. doi: 10.1038/nbt.1481. Epub 2008 Jul 20. Nat Biotechnol. 2008. PMID: 18641635 Free PMC article. - Discovery, biology and therapeutic potential of RNA interference, microRNA and antagomirs.
Yang M, Mattes J. Yang M, et al. Pharmacol Ther. 2008 Jan;117(1):94-104. doi: 10.1016/j.pharmthera.2007.08.004. Epub 2007 Sep 7. Pharmacol Ther. 2008. PMID: 17928059 Review. - Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54.
Chu CY, Rana TM. Chu CY, et al. PLoS Biol. 2006 Jul;4(7):e210. doi: 10.1371/journal.pbio.0040210. PLoS Biol. 2006. PMID: 16756390 Free PMC article.
Cited by
- Upregulated microRNA‑330‑3p promotes calcification in the bicuspid aortic valve via targeting CREBBP.
Zheng R, Liu H, Gu J, Ni B, Sun H, Guo Y, Su C, He K, Du J, Shao Y. Zheng R, et al. Mol Med Rep. 2020 Sep;22(3):2351-2363. doi: 10.3892/mmr.2020.11297. Epub 2020 Jul 6. Mol Med Rep. 2020. PMID: 32705274 Free PMC article. - The ADAR protein family.
Savva YA, Rieder LE, Reenan RA. Savva YA, et al. Genome Biol. 2012 Dec 28;13(12):252. doi: 10.1186/gb-2012-13-12-252. Genome Biol. 2012. PMID: 23273215 Free PMC article. Review. - Double-stranded regions are essential design components of potent inhibitors of RISC function.
Vermeulen A, Robertson B, Dalby AB, Marshall WS, Karpilow J, Leake D, Khvorova A, Baskerville S. Vermeulen A, et al. RNA. 2007 May;13(5):723-30. doi: 10.1261/rna.448107. Epub 2007 Mar 30. RNA. 2007. PMID: 17400817 Free PMC article. - Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver.
Elmén J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjärn M, Hansen JB, Hansen HF, Straarup EM, McCullagh K, Kearney P, Kauppinen S. Elmén J, et al. Nucleic Acids Res. 2008 Mar;36(4):1153-62. doi: 10.1093/nar/gkm1113. Epub 2007 Dec 23. Nucleic Acids Res. 2008. PMID: 18158304 Free PMC article. - The anti-miR21 antagomir, a therapeutic tool for colorectal cancer, has a potential synergistic effect by perturbing an angiogenesis-associated miR30.
Song MS, Rossi JJ. Song MS, et al. Front Genet. 2014 Jan 2;4:301. doi: 10.3389/fgene.2013.00301. eCollection 2014. Front Genet. 2014. PMID: 24427170 Free PMC article.
References
- Abrahante, J.E., Daul, A.L., Li, M., Volk, M.L., Tennessen, J.M., Miller, E.A., and Rougvie, A.E. 2003. The Caenorhabditis elegans hunchback-like gene lin-57/hbl-1 controls developmental time and is regulated by microRNAs. Dev. Cell 4: 625–637. - PubMed
- Ambros, V. 2003. MicroRNA pathways in flies and worms: Growth, death, fat, stress, and timing. Cell 113: 673–676. - PubMed
- Ambros, V., Lee, R.C., Lavanway, A., Williams, P.T., and Jewell, D. 2003. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr. Biol. 13: 807–818. - PubMed
- Aravin, A.A., Lagos-Quintana, M., Yalcin, A., Zavolan, M., Marks, D., Snyder, B., Gaasterland, T., Meyer, J., and Tuschl, T. 2003. The small RNA profile during Drosophila melanogaster development. Dev. Cell 5: 337–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials