Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis - PubMed (original) (raw)
Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis
Marisa Roberto et al. J Neurosci. 2004.
Abstract
The modulation of glutamatergic transmission by ethanol may contribute to ethanol intoxication, reinforcement, tolerance, and dependence. Therefore, we used in vitro electrophysiological and in vivo microdialysis techniques to investigate the effects of acute and chronic ethanol on glutamatergic transmission in the central nucleus of amygdala (CeA). Superfusion of 5-66 mM ethanol decreased compound glutamatergic EPSPs and EPSCs in CeA neurons, with half-maximal inhibition elicited by 14 mM ethanol. Ethanol (44 mM) decreased both non-NMDAR- and NMDAR-mediated EPSPs and EPSCs by 21%. Both the ethanol- and ifenprodil-induced depression of NMDAR-mediated EPSPs and EPSCs was enhanced in rats that received chronic ethanol treatment (CET). Ifenprodil also occluded the ethanol effect, suggesting that NR2B subunit-containing receptors may be involved. With local applications of NMDA, acute ethanol elicited a greater inhibition of NMDA currents in slices taken from CET (47%) compared with naive (30%) animals, suggesting that CET sensitizes NMDA receptors to ethanol. Acute ethanol also reduced paired pulse facilitation of EPSPs and EPSCs only in CET animals, suggesting acute ethanol-induced increase of glutamate release. This finding was supported by in vivo experiments showing that infusion of ethanol (0.1-1 M) via reverse microdialysis significantly increased glutamate release into the CeA dialysate but only after CET. Moreover, baseline CeA glutamate content was significantly higher in CET compared with naive animals. These combined findings suggest that CET and withdrawal lead to neuroadaptations of glutamatergic transmission at both presynaptic and postsynaptic sites in CeA, and glutamatergic synapses in CeA may play an important role in ethanol dependence.
Figures
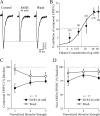
Figure 1.
Acute ethanol decreases excitatory synaptic transmission in CeA neurons. A, Superfusion of 44 m
m
ethanol decreased the amplitude of evoked compound glutamatergic EPSCs isolated by GABAergic blockers. B, Concentration-response relationship for ethanol inhibition of compound glutamatergic EPSP and EPSC amplitudes in CeA neurons (ethanol superfused for 7-10 min; number of cells in parentheses). The logistic curve is plotted by Origin Software (Microcal) using y = (_A_1 - _A_2)/[sqb]1 + (x/xo) * p + _A_2]. Parameters of the logistic curve were set at upper asymptote fixed at 21% and lower at 1%. The half-maximal inhibition was produced by 14 m
m
ethanol. Rate was fixed at 2.2, with “center” (half-maximal inhibition) unfixed. C, Decrease of compound EPSP and EPSC amplitudes elicited by 44 m
m
ethanol, averaged from 11 neurons. The mean EPSPs and EPSCs were significantly (p < 0.05) decreased by 26-39%. D, Pooled data from 17 neurons; in the presence of 30 μ
m
bicuculline, 1 μ
m
CGP 55845A, and 20 μ
m d
-AP-5, 44 m
m
ethanol significantly (p < 0.05) decreased the non-NMDAR-mediated EPSP and EPSC amplitudes by 15-26%.
Figure 2.
Acute ethanol decreases the amplitude of evoked NMDAR-mediated EPSPs and EPSCs. A, Representative NMDAR-mediated EPSCs evoked in the presence of 30 μ
m
bicuculline, 1 μ
m
CGP 55845A, 10 μ
m
CNQX, and low Mg2+ concentrations. Ethanol decreased the NMDAR-mediated EPSC amplitudes with partial recovery on washout. The subsequent addition of
d
-AP-5 completely blocked the EPSCs, implicating NMDARs. B, In slices from naive rats, superfusion of 44 m
m
ethanol significantly (p < 0.01; n = 17) reduced evoked NMDAR-mediated EPSPs and EPSCs by 15-20%.
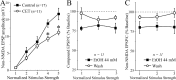
Figure 3.
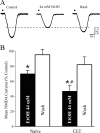
CET reduces baseline non-NMDA transmission but does not alter acute ethanol effects. A, I-O curves of non-NMDAR-mediated EPSP and EPSC amplitudes evoked by local stimulation. At higher stimulus strengths, the baseline response is significantly (*p < 0.05) reduced in slices taken from CET rats compared with controls. B, Acute ethanol significantly (p < 0.05) decreased by 20% compound EPSPs and EPSCs in slices from CET rats, to a similar extent as those from control slices. C, In neurons of CET rats, acute ethanol significantly (p < 0.05; n = 11) reduced non-NMDAR-mediated EPSPs and EPSCs (with partial recovery on washout) to a similar extent as in control slices.
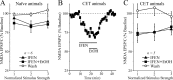
Figure 4.
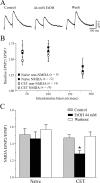
CET potentiates the inhibitory effect of acute ethanol on NMDAR-mediated EPSPs and EPSCs in CeA. A, Acute ethanol reduced NMDAR-mediated EPSP and EPSC amplitudes by 35-45% in neurons from CET rats (n = 15) compared with 15-20% in neurons from naive rats (Fig. 2 B), suggesting sensitization to acute ethanol. B, Ethanol superfusion significantly (p < 0.001; _n_ = 8) decreased evoked NMDAR-mediated EPSP amplitudes across the voltage range tested (with only partial recovery on washout), especially at the more depolarized potentials. _C_, In neurons from CET rats, evoked NMDAR-mediated EPSPs and EPSCs remained stable for >45 min of superfusion in the absence of ethanol.
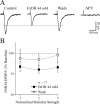
Figure 5.
The NR2B blocker ifenprodil occludes the depressant effect of acute ethanol. A, Bath application of 10 μ
m
ifenprodil (IFEN) for 10 min decreased the NMDAR-mediated EPSP and EPSC amplitudes (n = 6). Subsequent superfusion of 44 m
m
ethanol (EtOH) did not further affect the NMDAR-mediated EPSPs and EPSCs in these neurons taken from naive rats. B, Time course record depicting the sequential application of 10 μ
m
ifenprodil and 44 m
m
ethanol on NMDAR-mediated EPSC amplitudes in a neuron from a CET rat. C, Summary of the effect of ifenprodil and ethanol on five CeA neurons from CET rats. Ifenprodil significantly (p < 0.05) decreased NMDAR-mediated EPSP and EPSC amplitudes and prevented the ethanol effect, with full recovery on drug washout.
Figure 6.
Baseline PPF is unchanged after CET, and acute ethanol decreases PPF of NMDAR-mediated EPSPs and EPSCs but only after CET. A, Voltage records of NMDAR-mediated EPSPs in response to two stimuli 100 msec apart in a neuron from a CET rat. Acute ethanol reduced PPF. B, Pooled data of baseline PPF ratios (at 50, 100, and 180 msec interpulse intervals) of non-NMDAR- and NMDAR-mediated EPSPs and EPSCs in neurons of naive and CET rats. There was no significant difference between groups. C, Pooled data of PPF ratio expressed as the second EPSP or EPSC amplitude over the first EPSP or EPSC. Superfusion of ethanol significantly decreased the PPF ratio in neurons from CET (by 15%; *p < 0.05; n = 16) but not naive (n = 10) rats.
Figure 7.
Inward currents elicited by local application of NMDA are decreased by acute ethanol. A, Representative current records before, during, and after ethanol superfusion (8 min) from a neuron of a CET rat. Local pressure application (2 sec, 6 psi; bars above the records) of NMDA (10 m
m
in the pipette, applied every 2 min) in low-Mg2+ ACSF containing GABAergic blockers CNQX and 1 μ
m
TTX. B, In 4 neurons from naive rats, superfusion of 44 m
m
ethanol (5-10 min) significantly (*p < 0.05) decreased exogenous NMDA-induced currents by 30 ± 5%, with recovery on washout (95 ± 7% of control, 10-15 min). In four neurons from CET rats, 44 m
m
ethanol more strongly decreased NMDA currents by 53 ± 7% (*p < 0.001 compared with baseline; #p < 0.05 compared with ethanol-induced inhibition in control slices) with partial recovery on washout (to 82 ± 10% of control).
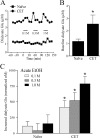
Figure 8.
In vivo dialysate levels of glutamate in CeA. A, Representative samples of dialysate glutamate levels from naive (squares) and CET (circles) rats depicting the effect of ethanol administration (0.1, 0.3, and 1.0
m
; bars). B, In CET rats, the baseline dialysate glutamate level was increased (2.6 ± 0.8 μ
m
; n = 7) compared with that in naive rats (1.2 ± 0.2 μ
m
; *p < 0.05; n = 11). C, In naive rats, local in vivo ethanol administration (0.1, 0.3, and 1.0
m
) into the CeA did not significantly (p > 0.05) alter local dialysate glutamate (Glu) levels (increase compared with baseline, 114 ± 72, 43 ± 110, and 153 ± 139, n
m
, respectively; n = 11). In agreement with our in vitro electrophysiological results, only in CET rats did local infusion of ethanol produce a significant dose-dependent increase in dialysate glutamate levels (increase compared with baseline, 413.3 ± 107.9, 522 ± 125, and 787.4 ± 116.0 nm, respectively; n = 7; *p < 0.05). Dialysates were normalized to baseline level.
Similar articles
- Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala.
Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Roberto M, et al. Neuropsychopharmacology. 2006 May;31(5):988-96. doi: 10.1038/sj.npp.1300840. Neuropsychopharmacology. 2006. PMID: 16052244 - Nociceptin/orphanin FQ decreases glutamate transmission and blocks ethanol-induced effects in the central amygdala of naive and ethanol-dependent rats.
Kallupi M, Varodayan FP, Oleata CS, Correia D, Luu G, Roberto M. Kallupi M, et al. Neuropsychopharmacology. 2014 Apr;39(5):1081-92. doi: 10.1038/npp.2013.308. Epub 2013 Oct 30. Neuropsychopharmacology. 2014. PMID: 24169802 Free PMC article. - Increased GABA release in the central amygdala of ethanol-dependent rats.
Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Roberto M, et al. J Neurosci. 2004 Nov 10;24(45):10159-66. doi: 10.1523/JNEUROSCI.3004-04.2004. J Neurosci. 2004. PMID: 15537886 Free PMC article. - Glutamatergic transmission in opiate and alcohol dependence.
Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, De Lecea L. Siggins GR, et al. Ann N Y Acad Sci. 2003 Nov;1003:196-211. doi: 10.1196/annals.1300.012. Ann N Y Acad Sci. 2003. PMID: 14684447 Review. - The central amygdala and alcohol: role of γ-aminobutyric acid, glutamate, and neuropeptides.
Roberto M, Gilpin NW, Siggins GR. Roberto M, et al. Cold Spring Harb Perspect Med. 2012 Dec 1;2(12):a012195. doi: 10.1101/cshperspect.a012195. Cold Spring Harb Perspect Med. 2012. PMID: 23085848 Free PMC article. Review.
Cited by
- Enhanced AMPA receptor activity increases operant alcohol self-administration and cue-induced reinstatement.
Cannady R, Fisher KR, Durant B, Besheer J, Hodge CW. Cannady R, et al. Addict Biol. 2013 Jan;18(1):54-65. doi: 10.1111/adb.12000. Epub 2012 Nov 6. Addict Biol. 2013. PMID: 23126443 Free PMC article. - Coordinated dynamic gene expression changes in the central nucleus of the amygdala during alcohol withdrawal.
Freeman K, Staehle MM, Vadigepalli R, Gonye GE, Ogunnaike BA, Hoek JB, Schwaber JS. Freeman K, et al. Alcohol Clin Exp Res. 2013 Jan;37 Suppl 1(0 1):E88-100. doi: 10.1111/j.1530-0277.2012.01910.x. Epub 2012 Jul 24. Alcohol Clin Exp Res. 2013. PMID: 22827539 Free PMC article. - Modulation of neuronal excitability by binge alcohol drinking.
Gimenez-Gomez P, Le T, Martin GE. Gimenez-Gomez P, et al. Front Mol Neurosci. 2023 Feb 14;16:1098211. doi: 10.3389/fnmol.2023.1098211. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36866357 Free PMC article. Review. - Ethanol exposure alters Alzheimer's-related pathology, behavior, and metabolism in APP/PS1 mice.
Day SM, Gironda SC, Clarke CW, Snipes JA, Nicol NI, Kamran H, Vaughan W, Weiner JL, Macauley SL. Day SM, et al. Neurobiol Dis. 2023 Feb;177:105967. doi: 10.1016/j.nbd.2022.105967. Epub 2022 Dec 16. Neurobiol Dis. 2023. PMID: 36535550 Free PMC article. - Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats.
Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL. Sari Y, et al. Alcohol Alcohol. 2011 May-Jun;46(3):239-46. doi: 10.1093/alcalc/agr023. Epub 2011 Mar 19. Alcohol Alcohol. 2011. PMID: 21422004 Free PMC article.
References
- Allgaier C (2002) Ethanol sensitivity of NMDA receptors. Neurochem Int 41: 377-382. - PubMed
- Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD (2003) Tyrosine dephosphorylation and ethanol inhibition of N-Methyl-d-aspartate receptor function. J Biol Chem 278: 11020-11025. - PubMed
- Broadbent J, Kampmueller KM, Koonse SA (2003) Expression of behavioral sensitization to ethanol by DBA/2J mice: the role of NMDA and non-NMDA glutamate receptors. Psychopharmacology 167: 225-234. - PubMed
- Calton JL, Wilson WA, Moore SD (1998) Magnesium-dependent inhibition of N-methyl-d-aspartate receptor-mediated synaptic transmission by ethanol. J Pharmacol Exp Ther 287: 1015-1019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AA012294/AA/NIAAA NIH HHS/United States
- AA12294/AA/NIAAA NIH HHS/United States
- DA13658/DA/NIDA NIH HHS/United States
- R01 DA003665/DA/NIDA NIH HHS/United States
- U24 AA013517/AA/NIAAA NIH HHS/United States
- R01 DA013658/DA/NIDA NIH HHS/United States
- DA03665/DA/NIDA NIH HHS/United States
- U01 AA013517/AA/NIAAA NIH HHS/United States
- P50 AA006420/AA/NIAAA NIH HHS/United States
- AA013517/AA/NIAAA NIH HHS/United States
- AA06420/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources