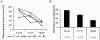X-chromosome inactivation patterns are unbalanced and affect the phenotypic outcome in a mouse model of rett syndrome - PubMed (original) (raw)
X-chromosome inactivation patterns are unbalanced and affect the phenotypic outcome in a mouse model of rett syndrome
Juan I Young et al. Am J Hum Genet. 2004 Mar.
Abstract
Rett syndrome (RTT), a neurodevelopmental disorder affecting mostly females, is caused by mutations in the X-linked gene encoding methyl-CpG-binding protein 2 (MeCP2). Although the majority of girls with classic RTT have a random pattern of X-chromosome inactivation (XCI), nonbalanced patterns have been observed in patients carrying mutant MECP2 and, in some cases, account for variability of phenotypic manifestations. We have generated an RTT mouse model that recapitulates all major aspects of the human disease, but we found that females exhibit a high degree of phenotypic variability beyond what is observed in human patients with similar mutations. To evaluate whether XCI influences the phenotypic outcome of Mecp2 mutation in the mouse, we studied the pattern of XCI at the single-cell level in brains of heterozygous females. We found that XCI patterns were unbalanced, favoring expression of the wild-type allele, in most mutant females. It is notable that none of the animals had nonrandom XCI favoring the mutant allele. To explore why the XCI patterns favored expression of the wild-type allele, we studied primary neuronal cultures from Mecp2-mutant mice and found selective survival of neurons in which the wild-type X chromosome was active. Quantitative analysis indicated that fewer phenotypes are observed when a large percentage of neurons have the mutant X chromosome inactivated. The study of neuronal XCI patterns in a large number of female mice carrying a mutant Mecp2 allele highlights the importance of MeCP2 for neuronal viability. These findings also raise the possibility that there are human females who carry mutant MECP2 alleles but are not recognized because their phenotypes are subdued owing to favorable XCI patterns.
Figures
Figure 1
Penetrance of phenotypes caused by the _Mecp2_308 allele. Each of the four panels shows an image of a mutant female mouse and of a wild-type female litter mate (inset). The percentage of animals of each sex manifesting a particular phenotype is shown below each panel. A, Representative image of a female with DF. The inset shows a wild-type litter mate with shiny and groomed fur. B, A picture taken at a shutter speed of 1 s reveals the tremor as a shadow effect over the mouse’s silhouette (arrows). The sharp inset picture of a wild-type mouse was taken at the same shutter speed. C, PLs that sometimes extend to the ears. Note also the disarranged appearance of the fur. Inset shows a normal eye in a wild-type litter mate. D, SFMs, inferred by looking at this picture taken with a shutter speed of 1 s. The arrow points to the forepaws of the mutant female, which have a shadow because of the rapid movements (see video by Shahbazian et al. [2002_a_] at the Neuron Web site).
Figure 2
Determination of XCI patterns in the cerebellum of Mecp2 308/X females by confocal laser scanning microscopy. Costaining with anti-calbindin antibody (red) and an antibody against the C-terminus of MeCP2 (green) (see lower diagram for antigen localization) allows us to label all PCs (calbindin) and identify neurons that express the wild-type Mecp2 allele (cells positive for calbindin and Mecp2 have yellow nuclei). Note that the majority of PCs are immunoreactive for the MeCP2 antibody. Arrows mark the few cells expressing mutant Mecp2 (nonimmunoreactive for α-MeCP2). Each panel is a representative example of immunofluorescence, visualized at increasingly higher magnifications. Scale bar = 20 μm.
Figure 3
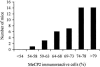
Distribution of XCI patterns in Mecp2 308/X female mice. Values on the _X_-axis represent the percentage of cells that have the wild-type X chromosome active, as assayed by coimmunofluorescence. Among a total of 45 female mice, none had <54% of cells with the wild-type chromosome as the active one.
Figure 4
XCI patterns in midbrain and cerebral cortex of Mecp2 308/X female mice. A and B, Double immunofluorescence for the detection of wild-type MeCP2 (green) and TH (red) was used to determine the XCI pattern in catecholaminergic neurons in the midbrain. Arrows denote cells that have the X chromosome bearing the mutant Mecp2 as the active one. C, Visualization of coimmunolabeling of Mecp2 (green) and parvalbumin (red) in the cerebral cortex by confocal microscopy. D, Same as in panel C, but a single focal section is shown, to demonstrate the definitive identification of cells expressing the wild-type (arrowhead) versus the mutant (arrow) Mecp2 allele. Scale bar = 20 μm.
Figure 5
Selective growth advantage of neurons expressing the wild-type allele when grown in culture. The proportion of neurons expressing either wild-type or truncated Mecp2 allele was determined by indirect immunofluorescence in hippocampal neurons from an E18 female _Mecp2_308/X embryo cultured for 4 or 7 d (A) and in neurons from mixed cultures derived from _Mecp2_308/Y and Mecp2+/Y embryos (1:1 ratio) cultured for 7 d (B). White bars = neurons expressing wild-type MeCP2. Gray bars = neurons expressing truncated MeCP2.
Figure 6
Correlation between degree of XCI skewing and phenotype. We divided the population of 45 females into three groups, according to their XCI patterns, and plotted them against the percentage of mice expressing a particular phenotype for each range of XCI patterns (A). Logistic regression analysis indicated that a unit increase in XCI was associated with a 16%, a 21%, a 9%, and a 12% decrease in the odds of Tr (
P<.01
), SFM (
P<.01
), DF (
P<.06
), and PL (
P<.02
), respectively. B, The cumulative number of phenotypes presented by the animals in each group plotted against the XCI groups. A significant difference was detected among XCI pattern groups (
P<.001
), and further testing indicated differences between groups 1 (54%–70%) and 2 (71%–77%) (
P<.05
), between groups 1 and 3 (>78%) (
P<.001
), and between groups 2 and 3 (
P<.02
).
Similar articles
- X-Chromosome inactivation ratios affect wild-type MeCP2 expression within mosaic Rett syndrome and Mecp2-/+ mouse brain.
Braunschweig D, Simcox T, Samaco RC, LaSalle JM. Braunschweig D, et al. Hum Mol Genet. 2004 Jun 15;13(12):1275-86. doi: 10.1093/hmg/ddh142. Epub 2004 Apr 28. Hum Mol Genet. 2004. PMID: 15115765 - Reduced proportion of Purkinje cells expressing paternally derived mutant Mecp2308 allele in female mouse cerebellum is not due to a skewed primary pattern of X-chromosome inactivation.
Watson CM, Pelka GJ, Radziewic T, Shahbazian MD, Christodoulou J, Williamson SL, Tam PP. Watson CM, et al. Hum Mol Genet. 2005 Jul 1;14(13):1851-61. doi: 10.1093/hmg/ddi191. Epub 2005 May 11. Hum Mol Genet. 2005. PMID: 15888476 - Rett syndrome: the complex nature of a monogenic disease.
Renieri A, Meloni I, Longo I, Ariani F, Mari F, Pescucci C, Cambi F. Renieri A, et al. J Mol Med (Berl). 2003 Jun;81(6):346-54. doi: 10.1007/s00109-003-0444-9. Epub 2003 May 16. J Mol Med (Berl). 2003. PMID: 12750821 Review. - Segregation of a totally skewed pattern of X chromosome inactivation in four familial cases of Rett syndrome without MECP2 mutation: implications for the disease.
Villard L, Lévy N, Xiang F, Kpebe A, Labelle V, Chevillard C, Zhang Z, Schwartz CE, Tardieu M, Chelly J, Anvret M, Fontès M. Villard L, et al. J Med Genet. 2001 Jul;38(7):435-42. doi: 10.1136/jmg.38.7.435. J Med Genet. 2001. PMID: 11432961 Free PMC article. - The role of X-chromosome inactivation in the manifestation of Rett syndrome.
Takagi N. Takagi N. Brain Dev. 2001 Dec;23 Suppl 1:S182-5. doi: 10.1016/s0387-7604(01)00362-x. Brain Dev. 2001. PMID: 11738869 Review.
Cited by
- Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome.
McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. McGill BE, et al. Proc Natl Acad Sci U S A. 2006 Nov 28;103(48):18267-72. doi: 10.1073/pnas.0608702103. Epub 2006 Nov 15. Proc Natl Acad Sci U S A. 2006. PMID: 17108082 Free PMC article. - Very mild cases of Rett syndrome with skewed X inactivation.
Huppke P, Maier EM, Warnke A, Brendel C, Laccone F, Gärtner J. Huppke P, et al. J Med Genet. 2006 Oct;43(10):814-6. doi: 10.1136/jmg.2006.042077. Epub 2006 May 11. J Med Genet. 2006. PMID: 16690727 Free PMC article. - Losing Dnmt3a dependent methylation in inhibitory neurons impairs neural function by a mechanism impacting Rett syndrome.
Lavery LA, Ure K, Wan YW, Luo C, Trostle AJ, Wang W, Jin H, Lopez J, Lucero J, Durham MA, Castanon R, Nery JR, Liu Z, Goodell M, Ecker JR, Behrens MM, Zoghbi HY. Lavery LA, et al. Elife. 2020 Mar 11;9:e52981. doi: 10.7554/eLife.52981. Elife. 2020. PMID: 32159514 Free PMC article. - Neuronal maturation defect in induced pluripotent stem cells from patients with Rett syndrome.
Kim KY, Hysolli E, Park IH. Kim KY, et al. Proc Natl Acad Sci U S A. 2011 Aug 23;108(34):14169-74. doi: 10.1073/pnas.1018979108. Epub 2011 Aug 1. Proc Natl Acad Sci U S A. 2011. PMID: 21807996 Free PMC article. - Genotype-specific effects of Mecp2 loss-of-function on morphology of Layer V pyramidal neurons in heterozygous female Rett syndrome model mice.
Rietveld L, Stuss DP, McPhee D, Delaney KR. Rietveld L, et al. Front Cell Neurosci. 2015 Apr 20;9:145. doi: 10.3389/fncel.2015.00145. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25941473 Free PMC article.
References
Electronic-Database Information
- Neuron, http://www.neuron.org/content/full/35/2/243/DC1 (for Shahbazian et al. video)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for RTT) - PubMed
- RettBASE: IRSA MECP2 Variation Database, http://mecp2.chw.edu.au/ - PubMed
References
- Adler DA, Quaderi NA, Brown SD, Chapman VM, Moore J, Tate P, Disteche CM (1995) The X-linked methylated DNA binding protein, Mecp2, is subject to X inactivation in the mouse. Mamm Genome 6:491–492 - PubMed
- Amir RE, Van den Veyver IB, Schultz R, Malicki DM, Tran CQ, Dahle EJ, Philippi A, Timar L, Percy AK, Motil KJ, Lichtarge O, Smith EO, Glaze DG, Zoghbi HY (2000) Influence of mutation type and X chromosome inactivation on Rett syndrome phenotypes. Ann Neurol 47:670–67910.1002/1531-8249(200005)47:5<670::AID-ANA20>3.3.CO;2-6 - DOI - PubMed
- Armstrong DD, Deguchi K, Antallfy B (2003) Survey of MeCP2 in the Rett syndrome and the non-Rett syndrome brain. J Child Neurol 18:653–660 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases