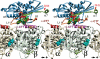Modulation of kinesin binding by the C-termini of tubulin - PubMed (original) (raw)
Modulation of kinesin binding by the C-termini of tubulin
Georgios Skiniotis et al. EMBO J. 2004.
Abstract
The flexible tubulin C-terminal tails (CTTs) have recently been implicated in the walking mechanism of dynein and kinesin. To address their role in the case of conventional kinesin, we examined the structure of kinesin-microtubule (MT) complexes before and after CTT cleavage by subtilisin. Our results show that the CTTs directly modulate the motor-tubulin interface and the binding properties of motors. CTT cleavage increases motor binding stability, and kinesin appears to adopt a binding conformation close to the nucleotide-free configuration under most nucleotide conditions. Moreover, C-terminal cleavage results in trapping a transient motor-ADP-MT intermediate. Using SH3-tagged dimeric and monomeric constructs, we could also show that the position of the kinesin neck is not affected by the C-terminal segments of tubulin. Overall, our study reveals that the tubulin C-termini define the stability of the MT-kinesin complex in a nucleotide-dependent manner, and highlights the involvement of tubulin in the regulation of weak and strong kinesin binding states.
Figures
Figure 1
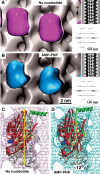
Nucleotide-dependent structural changes in monomeric kinesin motor domains. (A) 3D reconstruction of MTs decorated with monomeric rat kinesin rK354 in the absence of nucleotide (magenta), and (B) in the presence of AMP-PNP (cyan). Corresponding lateral projections and diffraction patterns are shown in the insets. In both reconstructions, decoration of tubulin dimers by motor domains was complete (see Figure 3). Docking of the X-ray structure into the EM-derived envelopes reveals an approximately 12° anticlockwise head rotation after transition from a nucleotide-free state (C) to an AMP-PNP state (D).
Figure 2
Statistical 3D difference mapping between monomeric kinesins in the presence or absence of the tubulin C-terminus. Difference volumes include areas with a significance of >99%. The left panels show sections through the reconstructions along the red lines indicated on the right. (A) On untreated MTs, comparison between nucleotide-free and AMP-PNP states revealed a significant density difference (magenta) at the motor–tubulin interface (for atomic details, see Figure 5). (B) The differences detected under native conditions shown in (A) disappear when subtilisin-treated MTs are used. (C) Comparing subtilisin-treated versus untreated kinesin–MT complexes in the presence of AMP-PNP reveals a similar difference volume (green) as found in (A), indicating that the AMP-PNP-bound state of kinesin approximates a no-nucleotide state once the CTTs are cleaved off.
Figure 3
Interaction of monomeric kinesin motor domains with subtilisin-treated MTs. MT pelleting assays and SDS–PAGE of subtilisin-treated (+subt.) and native (−subt.) MTs incubated with monomeric rK354 (A) in the absence of nucleotide and (B) the presence of AMP-PNP, or (C) excess ADP (s=supernatant, p=pellet). Subtilisin-treated MTs reveal the two characteristic lower tubulin bands as indicated. Kinesin motor domains in the absence of nucleotides (A) and in the presence of AMP-PNP (B) bind to both native and subtilisin-treated MTs at comparable amounts, indicating full decoration of the MT surface. (C) In the presence of excess ADP (20 mM), subtilisin treatment clearly increases the amount of kinesin motor domains in the pellet. (D) SDS–PAGE, comparing native and subtilisin-treated tubulin under strongly separating conditions, reveals the molecular mass shifts for both α- and β-tubulin (assignments of α- and β-tubulin are according to Bhattacharyya et al, 1985).
Figure 4
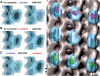
Monomeric kinesin binding in the presence of excess ADP on native or subtilisin-treated MTs. (A) The presence of excess ADP (20 mM) strongly reduces the MT decoration efficiency of kinesin motor domains. Cryo-EM images of MTs under these conditions do not show any signs of motor decoration (inset: averaged projections), which is reflected in the absence of a strong 1/8 nm layer line, corresponding to the axial tubulin dimer repeat. (B) After cleavage of CTTs, ADP–rK354 decorates MTs very efficiently. Cryo-EM images reveal a much rougher outer MT surface, giving rise to a strong 1/8 nm reflection (one motor head per αβ-tubulin dimer). In 3D –reconstructions, the motor heads (green) are clearly visible on the MT portion. Scale bars on micrographs=20 nm.
Figure 5
The C-terminal tail of β-tubulin locates at the vicinity of the switch II cluster. Stereo image of the kinesin–tubulin complex as derived from molecular docking of the atomic-resolution structures into cryo-EM density envelopes of MT–kinesin complexes. The statistically relevant (>99%) density difference found between AMP-PNP and nucleotide-free states is shown as a red wire frame (located behind α4 and α5). The same location was found for a density difference between MT–kinesin complexes in the AMP-PNP state, before and after subtilisin treatment (see Figure 2). The C-terminus of β-tubulin locates in close proximity to loop 11, α4, loop 12 and α5 of kinesin. ‘SH3' marks the insertion sites of SH3 density labels shown in Figure 6.
Figure 6
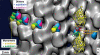
Tubulin C-terminal cleavage does not affect the positions of the neck region in monomeric and dimeric kinesins. The kinesin neck region position was probed by monomeric and dimeric constructs carrying an SH3 domain at the transitions between the neck-linker and the neck coiled-coil helix α7. In the presence of AMP-PNP, the kinesin neck is found in a stable position towards the plus-end in both complexes with native and subtilisin-treated MTs. However, its position in dimers (blue, before treatment; magenta, after treatment) locates closer to the tubulin surface than in monomers (yellow, before treatment; green, after treatment). Hence, for both monomeric and dimeric constructs, their positions were not affected by the presence or absence of the β-tubulin C-terminus. The atomic model of the docked dimeric rK379–SH3 construct is shown on the right (see also Skiniotis et al, 2003).
Figure 7
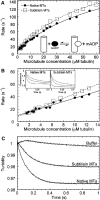
Characterization of the MT–kinesin complexes. (A) Native MT–rK354 and subtilisin-treated MT–rK354 complexes (0.1 μM rK354 plus taxol-stabilized MTs at 8 μM tubulin) were preformed and incubated with [α32P]ATP. The steady-state ATPase is shown as a function of MgATP concentration: native MT, _k_cat=61.9±1.2 s−1 and _K_m,ATP=77.4±4.2 μM; subtilisin-treated MT, _k_cat=61.2±0.9 s−1 and _K_m,ATP=52.8±2.5 μM. (B) Native MT–rK354 and subtilisin-treated MT–rK354 complexes were preformed with 0.25 μM rK354 plus taxol-stabilized MTs (0−6 μM tubulin) and reacted with 1 mM [α32P]ATP. The steady-state ATPase parameters were determined for native MTs (_k_cat=67.8±2.3 s−1 and _K_1/2,MT=0.51±0.06 μM) and subtilisin-treated MTs (_k_cat=63.3±1.9 s−1 and _K_1/2,MT=0.21±0.03 μM). (C) Equilibrium binding of 2 μM rK354 with varying concentrations of native and subtilisin-treated MTs (0−4 μM tubulin) in the absence of nucleotide. The fraction of rK354 in the MT pellet was plotted as a function of the total MT concentration. For native MTs, _K_d,MT=0.44±0.13 μM with maximum fractional binding at 1.1±0.1. For subtilisin-treated MTs, _K_d,MT=0.46±0.17 μM with maximum fractional binding at 1.2±0.1. (D) Equilibrium binding of 4 μM rK354, varying concentrations of [α32P]AMP-PNP (0−600 μM), and native or subtilisin-treated MTs (5 μM tubulin). The concentration of [α32P]AMP-PNP that partitioned with the MT–rK354 complexes was plotted as a function of MgAMP-PNP concentration. _K_d,AMP−PNP was 142±49 μM for native MT–rK354 complexes with the maximal AMP-PNP binding at 3.83±0.52 μM sites. For subtilisin-treated MTs, _K_d,AMP-PNP=142±53 μM with maximal AMP-PNP binding at 3.93±0.58 μM sites. (E) Equilibrium binding of 3 μM rK354, varying concentrations of [α32P]ADP (0−400 μM), and native or subtilisin-treated MTs (4 μM tubulin). The concentration of [α32P]ADP that partitioned with the MT–rK354 complex was plotted as a function of MgADP concentration. _K_d,ADP=99±20 μM for the native MT–rK354 complex with maximal ADP binding at 100% sites. For the subtilisin-treated MT–rK354 complex, _K_d,ADP=26±5 μM with maximal ADP binding at 100% sites. (F) Partitioning of 2 μM rK354 with 3 μM MTs as a function of MgADP concentration. (G) Summary of the MT–kinesin constants based on 3–7 independent experiments for each. The conditions were 80 mM PIPES (pH 6.8) with NaOH, 1 mM MgCl2, 1 mM EGTA at 25°C.
Figure 8
Pre-steady-state kinetics of the MT–rK354 complexes. (A) MantADP release kinetics. The rK354–mantADP complex was preformed and rapidly mixed in the stopped-flow instrument with varying concentrations of native and subtilisin-treated MTs+1 mM MgATP (final concentrations after mixing, 1.5 μM rK354+3 μM mantADP for MTs from 1.56–2.8 μM and 3 μM rK354+6 μM mantADP for MTs from 3.125–60 μM). Representative transients are shown in the inset of (B) (3.125, 6.25, 12.5 μM MTs from top to bottom transient). Each transient was fit to an exponential function, and the rate of the fluorescence decrease was plotted as a function of MT concentration. The fit of the data to hyperbolae provided the maximum rate constant for mantADP release for native MTs (_k_max=239±18 s−1, _K_1/2,MT=69.7±9.1 μM) and subtilisin MTs (_k_max=242±11 s−1, _K_1/2,MT=54.1±5.3 μM). (B) The data at low MT concentrations shown in (A) were re-plotted here to determine the second-order rate constant for MT association. The slope predicts the minimum estimate for MT association. Native MTs: 2.75±0.16 μM−1 s−1; subtilisin MTs: 3.69±0.11 μM−1 s−1. (C) MT–rK354 complexes were preformed and rapidly mixed with MgATP+KCl (final concentrations after mixing: 4 μM rK354, 5 μM MTs, 1 mM MgATP, 50 mM KCl in BRB80 buffer). The transients were each normalized to begin at 1 V to compare amplitudes. Note that the buffer control with 50 mM KCl exhibited no significant change in turbidity. Native MTs: _k_obs=7.9±0.02 s−1; subtilisin MTs: _k_obs=5.9±0.02 s−1.
Figure 9
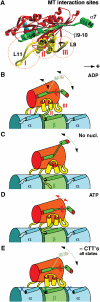
Schematic model for kinesin interaction with MTs at different nucleotide states and in dependence of the CTTs. (A) The kinesin–MT interface consists of three different clusters, loop 11 (I), α4-loop12-α5 (II), and loop 8 (III). (B) In the presence of ADP, the affinity of kinesin to MTs is rather weak and probably only mediated through loop 11 (Asenjo et al, 2003), which does not seem to be influenced by the CTTs. (C) Upon ADP release, binding sites II and III become more effective through rearrangements in the switch-II region. (D) Upon ATP uptake, the neck-linker (β9–10, α7) locks in its plus-end pointing position and binding sites II and III experience a stronger interference from the β-tubulin C-terminus. It may be possible that α7 interferes with the α-tubulin C-terminus, rendering the head more mobile. (E) Absence of CTTs renders all binding regions equally rigid through all nucleotide states.
Similar articles
- A new look at the microtubule binding patterns of dimeric kinesins.
Hoenger A, Thormählen M, Diaz-Avalos R, Doerhoefer M, Goldie KN, Müller J, Mandelkow E. Hoenger A, et al. J Mol Biol. 2000 Apr 14;297(5):1087-103. doi: 10.1006/jmbi.2000.3627. J Mol Biol. 2000. PMID: 10764575 - The yeast kinesin-5 Cin8 interacts with the microtubule in a noncanonical manner.
Bell KM, Cha HK, Sindelar CV, Cochran JC. Bell KM, et al. J Biol Chem. 2017 Sep 1;292(35):14680-14694. doi: 10.1074/jbc.M117.797662. Epub 2017 Jul 12. J Biol Chem. 2017. PMID: 28701465 Free PMC article. - The E-hook of tubulin interacts with kinesin's head to increase processivity and speed.
Lakämper S, Meyhöfer E. Lakämper S, et al. Biophys J. 2005 Nov;89(5):3223-34. doi: 10.1529/biophysj.104.057505. Epub 2005 Aug 12. Biophys J. 2005. PMID: 16100283 Free PMC article. - Interaction of kinesin motors, microtubules, and MAPs.
Marx A, Müller J, Mandelkow EM, Hoenger A, Mandelkow E. Marx A, et al. J Muscle Res Cell Motil. 2006;27(2):125-37. doi: 10.1007/s10974-005-9051-4. Epub 2005 Dec 17. J Muscle Res Cell Motil. 2006. PMID: 16362723 Review. - Lucky 13-microtubule depolymerisation by kinesin-13 motors.
Moores CA, Milligan RA. Moores CA, et al. J Cell Sci. 2006 Oct 1;119(Pt 19):3905-13. doi: 10.1242/jcs.03224. J Cell Sci. 2006. PMID: 16988025 Review.
Cited by
- Paclitaxel-conjugated PAMAM dendrimers adversely affect microtubule structure through two independent modes of action.
Cline EN, Li MH, Choi SK, Herbstman JF, Kaul N, Meyhöfer E, Skiniotis G, Baker JR, Larson RG, Walter NG. Cline EN, et al. Biomacromolecules. 2013 Mar 11;14(3):654-64. doi: 10.1021/bm301719b. Epub 2013 Feb 21. Biomacromolecules. 2013. PMID: 23391096 Free PMC article. - Cholesterol in the cargo membrane amplifies tau inhibition of kinesin-1-based transport.
Li Q, Ferrare JT, Silver J, Wilson JO, Arteaga-Castaneda L, Qiu W, Vershinin M, King SJ, Neuman KC, Xu J. Li Q, et al. Proc Natl Acad Sci U S A. 2023 Jan 17;120(3):e2212507120. doi: 10.1073/pnas.2212507120. Epub 2023 Jan 10. Proc Natl Acad Sci U S A. 2023. PMID: 36626558 Free PMC article. - Mechanisms of Chromosome Congression during Mitosis.
Maiato H, Gomes AM, Sousa F, Barisic M. Maiato H, et al. Biology (Basel). 2017 Feb 17;6(1):13. doi: 10.3390/biology6010013. Biology (Basel). 2017. PMID: 28218637 Free PMC article. Review. - Tether-scanning the kinesin motor domain reveals a core mechanical action.
Sumiyoshi R, Yamagishi M, Furuta A, Nishizaka T, Furuta K, Cross RA, Yajima J. Sumiyoshi R, et al. Proc Natl Acad Sci U S A. 2024 Jul 23;121(30):e2403739121. doi: 10.1073/pnas.2403739121. Epub 2024 Jul 16. Proc Natl Acad Sci U S A. 2024. PMID: 39012822 Free PMC article. - Direct involvement of the isotype-specific C-terminus of beta tubulin in ciliary beating.
Vent J, Wyatt TA, Smith DD, Banerjee A, Ludueña RF, Sisson JH, Hallworth R. Vent J, et al. J Cell Sci. 2005 Oct 1;118(Pt 19):4333-41. doi: 10.1242/jcs.02550. Epub 2005 Sep 13. J Cell Sci. 2005. PMID: 16159957 Free PMC article.
References
- Asenjo AB, Krohn N, Sosa H (2003) Configuration of the two kinesin motor domains during ATP hydrolysis. Nat Struct Biol 10: 836–842 - PubMed
- Beuron F, Hoenger A (2001) Structural analysis of the microtubule–kinesin complex by Cryo-electron microscopy. In Kinesin Protocols, Vernos I (ed) Totowa, NJ: Humana Press Inc. pp 235–254 - PubMed
- Bhattacharyya B, Sackett DL, Wolff J (1985) Tubulin, hybrid dimers, and tubulin S. Stepwise charge reduction and polymerization. J Biol Chem 260: 10208–10216 - PubMed
- Crevel IM, Lockhart A, Cross RA (1996) Weak and strong states of kinesin and ncd. J Mol Biol 257: 66–76 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources