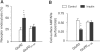Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD - PubMed (original) (raw)
. 2004 Mar 10;23(5):1040-50.
doi: 10.1038/sj.emboj.7600126. Epub 2004 Feb 19.
William Ju, Lidong Liu, Michael Wyszynski, Sang Hyoung Lee, Anthone W Dunah, Changiz Taghibiglou, Yushan Wang, Jie Lu, Tak Pan Wong, Morgan Sheng, Yu Tian Wang
Affiliations
- PMID: 14976558
- PMCID: PMC380981
- DOI: 10.1038/sj.emboj.7600126
Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD
Gholamreza Ahmadian et al. EMBO J. 2004.
Abstract
The alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) subtype of glutamate receptors is subject to functionally distinct constitutive and regulated clathrin-dependent endocytosis, contributing to various forms of synaptic plasticity. In HEK293 cells transiently expressing GluR1 or GluR2 mutants containing domain deletions or point mutations in their intracellular carboxyl termini (CT), we found that deletion of the first 10 amino acids (834-843) selectively reduced the rate of constitutive AMPA receptor endocytosis, whereas truncation of the last 15 amino acids of the GluR2 CT, or point mutation of the tyrosine residues in this region, only eliminated the regulated (insulin-stimulated) endocytosis. Moreover, in hippocampal slices, both insulin treatment and low-frequency stimulation (LFS) specifically stimulated tyrosine phosphorylation of the GluR2 subunits of native AMPA receptors, and the enhanced phosphorylation appears necessary for both insulin- and LFS-induced long-term depression of AMPA receptor-mediated excitatory postsynaptic currents. Thus, our results demonstrate that constitutive and regulated AMPA receptor endocytosis requires different sequences within GluR CTs and tyrosine phosphorylation of GluR2 CT is required for the regulated AMPA receptor endocytosis and hence the expression of certain forms of synaptic plasticity.
Figures
Figure 1
Construction of GluR2 internal deletion or CT truncation mutants. (A) Putative topology of AMPA receptor GluR2 subunit with HA tag inserted in the N-terminal extracellular domain. The expanded region shows the amino-acid sequence of the CT of GluR2. Sequences conserved among GluR1–4 (conserved) and those involved in its interaction with NSF (NSF) or PDZ domain-containing proteins (PDZ) are underlined. (B) CT sequences of internal deletion or truncation mutants of the full-length HA-tagged or nontagged GluR2 subunit. (C) Expression levels of the constructs following transient transfection into HEK293 cells were determined by cell-ELISA assays using an anti-HA antibody for HA-tagged constructs, or an anti-GluR2 subunit antibody for the non-HA-tagged construct, under permeabilized conditions. The level of expression was normalized to the expression level of HA–GluR2.
Figure 2
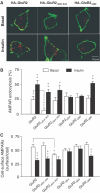
Effects of GluR2 CT mutations on endocytosis and cell-surface expression of AMPA receptors. (A) Representative confocal images of HEK293 cells transiently transfected with HA-tagged GluR2 or GluR2 mutants (as indicated). Transfected cells were prelabeled with anti-HA antibody and then receptor endocytosis was evaluated under basal conditions (constitutive endocytosis) or following insulin stimulation (0.5 μM, 10 min; regulated endocytosis). Cell-surface receptors were stained with FITC (green) under nonpermeant conditions and internalized receptors were subsequently stained with Cy3 (red) after cell permeabilization. (B) Quantitation of the changes in constitutive (basal) and regulated (insulin) endocytosis of GluR2 and its various mutants using a colorimetric ELISA assay with prelabeled cells following the internalization of the receptors over 30 min (% AMPAR endocytosis=100%−remaining cell-surface receptors/total number of receptors; _n_=6). Control: internalization measured in cells at 4°C without any 37°C exposure. (C) Cell-surface AMPA receptors in HEK293 cells transiently expressing GluR2 and its various mutants were quantitated using colorimetric cell-ELISA-based cell-surface receptor assays (_n_=6). Statistical comparisons were made between basal and insulin-treated conditions, except where indicated by lines. *P<0.05, **P<0.01.
Figure 3
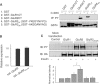
Insulin increases phosphorylation of tyrosine residues within the GluR2 CT region. (A) In vitro tyrosine phosphorylation of the GluR2 CT. GST fusion proteins of the GluR1 CT (GST–GluR1CT), the GluR2 CT (GST–GluR2CT), residues 869–876 (YKEGYNVYG) of the GluR2 CT (GST–GluR23Y) and the same amino-acid stretch of the GluR2 CT with its tyrosine residues replaced by alanines (GST–GluR23A), along with GST as control, were incubated in the absence (−) or presence (+) of active recombinant pp60 c-Src. Phosphorylation products were immunoblotted using an antiphosphotyrosine antibody (top panel). Ponceau S staining of the same blot showed that a similar amount of GST fusion protein was used in each of the reactions (lower panel). (B) Expression levels of HA–GluR2 and HA–GluR23Y-3A (where tyrosines 869, 873 and 876 were mutated to alanines) 48 h after transient transfection into HEK293 cells were determined by a cell-ELISA assay using permeabilized cells. (C) HEK293 cells transiently transfected with HA–GluR1, HA–GluR2 or HA–GluR23Y-3A, along with empty vector (mock transfection) as control. After 48 h, the cells were treated with or without 0.5 μM insulin for 10 min. The lysates were then subjected to immunoprecipitation with an anti-HA antibody under denaturing conditions and immunoblotting with an antiphosphotyrosine antibody (top blot; IB: PY). The same blot was stripped and re-immunoblotted with the anti-HA antibody to ensure similar immunoprecipitation efficiency in all individual experiments (lower blot; IB: HA). The bar graph at the bottom summarizes data from five individual experiments. Values are normalized to GluR1 without insulin treatment. *P<0.05.
Figure 4
The tyrosine cluster in the GluR2 CT is required for regulated, but not constitutive, AMPA receptor endocytosis in HEK293 cells. (A) Colorimetric cell-ELISA receptor endocytosis assays were performed with (insulin) or without (control) stimulation (see Figure 2) on HEK293 cells transiently transfected with wild-type HA–GluR2 subunit or HA–GluR23Y-3A. (B) Colorimetric cell-ELISA cell-surface receptor assay results of HEK293 cells transfected and treated as in (A). Results were obtained from six experiments for each individual group. **P<0.01.
Figure 5
The GluR2 subunit of native AMPA receptors is specifically tyrosine phosphorylated in mature neurons. Membrane proteins from adult rat cerebral cortex were solubilized and immunoprecipitated (IP) using an antiphosphotyrosine antibody (anti-PY; PY20) alone (−) or in the presence of 5 mM phosphotyrosine (PY) or 5 mM phosphoserine (PS) competitor following SDS (denaturing) or DOC (nondenaturing) extraction. The immunoprecipitates were immunoblotted (IB) with antibodies raised against the indicated proteins. Total phosphotyrosine proteins were probed with RC20H to measure the efficiency of precipitation of total phosphotyrosine proteins (bottom row); only the ∼180 kDa band is shown. Numbers represent percentage (%) of input (whole lysates) or immunoprecipitate loaded.
Figure 6
Insulin stimulates tyrosine phosphorylation of GluR2 and long-lasting depression of AMPA receptor-mediated synaptic transmission. (A) Tissue homogenates from hippocampal slices treated with (INS) or without (basal) 0.5 μM insulin for 10 min were immunoprecipitated with anti-GluR1 or -GluR2 antibodies under denaturing conditions (IP: GluR1 or GluR2) and immunoblotted using an antiphosphotyrosine antibody (IB: PY). The blot was then sequentially stripped and reprobed with anti-GluR2 (IB: GluR2) or anti-GluR1 (IB: GluR1) antibodies. Densitometric quantitation expressed as the ratio of phosphorylated GluR2 to total GluR2 from three separate experiments is summarized in the histogram on the right. **P<0.01. (**B**) EPSCs were recorded in CA1 neurons from hippocampal slices using whole-cell recordings under the voltage-clamp mode at a holding potential of −60 mV. Normalized EPSCs (EPSCt/EPSC5; the amplitude of EPSCs (EPSC5) is usually stabilized after 5 min of recording) are plotted from neurons recorded with pipettes containing standard intracellular solution (control, _n_=7) or intracellular solution supplemented with GluR23Y (100 μg/ml; _n_=5), GST–A869KEGA873NVA876G (GluR23A; 100 μg/ml; _n_=6) or the tyrosine-phosphorylated synthetic peptide Y_p_KEGY_p_NVY_p_G (GluR23Y-_P_; 50 μg/ml; _n_=6). Time zero is defined as the time point at which the first EPSC was evoked (typically within 1–2 min of the initiation of whole-cell recording). Representative EPSCs averaged from four individual recordings before (basal) or 10 min following application of insulin (INS; 0.5 μM) are shown on the left. Histogram at the bottom right summarizes the depression of the EPSC amplitude after 20 min of insulin stimulation. There was no statistical difference in control versus GST–GluR23A groups or GST–GluR23Y versus GluR23Y-_P_ (_P_>0.5), but there was a significant difference between control versus GST–GluR23Y and control versus GluR23Y-P (P<0.001).
Figure 7
Tyrosine phosphorylation of the GluR2 subunit is required for LFS-induced hippocampal CA1 LTD. (A) Homogenates of control or LFS-treated hippocampal slices were immunoprecipitated with anti-GluR1 or GluR2 antibodies and sequentially probed with antiphosphotyrosine (PY), anti-GluR1 (GluR1) and anti-GluR2 antibodies (GluR2) as described for Figure 6. The lane marked M contains molecular weight standards. The results of three individual experiments are summarized in the bar graph. **P<0.01. (B) Representative responses are shown on the left. The graphs on the right depict normalized EPSCs (EPSCt/EPSC5) from neurons recorded with pipettes containing standard intracellular solution (control, _n_=7) or intracellular solution supplemented with GluR23Y (_n_=6) or GluR23A (_n_=7). The LFS was delivered during the time period indicated by the black horizontal bar. Histogram on the bottom right summarizes the depression of EPSC amplitude 20 min after LFS. The control group was significantly different from the GST–GluR23Y group (P<0.001), but not the GST–GluR23A group.
Figure 8
GluR2 CT tyrosine residues are critical for insulin-stimulated endocytosis of AMPA receptors in cultured hippocampal neurons. (A) Representative double-label images of surface and intracellular expression of HA–GluR2 wild type (WT) and HA–GluR23Y-3A (3Y-3A) in transiently transfected hippocampal neurons. (B) Quantitation of surface to intracellular ratio of HA–GluR23Y-3A, normalized to WT (_n_=10 cells for each construct). (C) Representative images of neurons stained for surface and internalized WT and 3Y-3A constructs, after 10 min incubation in either conditioned medium (control), or medium containing 100 μM AMPA (AMPA) or 0.5 μM insulin (insulin). (D) Quantitation of internalization assays, measured as the ratio of internalized to surface fluorescence (internalization index), normalized to WT 10 min control. Histograms show mean±s.e.m. (_n_=8–10 for each condition). **P<0.001 compared with WT plus insulin; *P<0.05 compared with WT control. (E, F) Cultured hippocampal neurons were treated with (insulin; 0.5 μM) or without (basal) insulin in the presence of either GluR23Y (1 μM), PEP-1 carrier peptide (20 μM) or both. (E) Cell-surface expression of native AMPA receptors was measured by a colorimetric cell-ELISA assay using an antibody against the N-terminal extracellular domain of native AMPA receptor GluR2 subunits (_n_=6). (F) The level of tyrosine phosphorylation of native AMPA receptor GluR2 was determined by immunoprecipitation of cell lysates with the same anti-GluR2 antibody and immunoblotting with an antiphosphotyrosine antibody (left). Densitometric quantitation expressed as percentage of control is summarized in the histogram on the right (_n_=3). *P<0.05.
Similar articles
- Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization.
Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Man HY, et al. Neuron. 2000 Mar;25(3):649-62. doi: 10.1016/s0896-6273(00)81067-3. Neuron. 2000. PMID: 10774732 - Tyrosine phosphorylation of the GluR2 subunit is required for long-term depression of synaptic efficacy in young animals in vivo.
Fox CJ, Russell K, Titterness AK, Wang YT, Christie BR. Fox CJ, et al. Hippocampus. 2007;17(8):600-5. doi: 10.1002/hipo.20302. Hippocampus. 2007. PMID: 17534972 - An investigation into signal transduction mechanisms involved in insulin-induced long-term depression in the CA1 region of the hippocampus.
Huang CC, Lee CC, Hsu KS. Huang CC, et al. J Neurochem. 2004 Apr;89(1):217-31. doi: 10.1111/j.1471-4159.2003.02307.x. J Neurochem. 2004. PMID: 15030406 - Phosphorylation of AMPA receptors: mechanisms and synaptic plasticity.
Wang JQ, Arora A, Yang L, Parelkar NK, Zhang G, Liu X, Choe ES, Mao L. Wang JQ, et al. Mol Neurobiol. 2005 Dec;32(3):237-49. doi: 10.1385/MN:32:3:237. Mol Neurobiol. 2005. PMID: 16385140 Review. - Modification of AMPA receptor clustering regulates cerebellar synaptic plasticity.
Hirai H. Hirai H. Neurosci Res. 2001 Mar;39(3):261-7. doi: 10.1016/s0168-0102(00)00237-6. Neurosci Res. 2001. PMID: 11248365 Review.
Cited by
- Diabetes and dementia - the two faces of Janus.
Papazafiropoulou AK, Koros C, Melidonis A, Antonopoulos S. Papazafiropoulou AK, et al. Arch Med Sci Atheroscler Dis. 2020 Jul 21;5:e186-e197. doi: 10.5114/amsad.2020.97433. eCollection 2020. Arch Med Sci Atheroscler Dis. 2020. PMID: 32832719 Free PMC article. - Affective and Cognitive Impairments in Rodent Models of Diabetes.
Palazzo E, Marabese I, Boccella S, Belardo C, Pierretti G, Maione S. Palazzo E, et al. Curr Neuropharmacol. 2024;22(8):1327-1343. doi: 10.2174/1570159X22666240124164804. Curr Neuropharmacol. 2024. PMID: 38279738 Free PMC article. Review. - Actin polymerization-dependent increase in synaptic Arc/Arg3.1 expression in the amygdala is crucial for the expression of aversive memory associated with drug withdrawal.
Liu Y, Zhou QX, Hou YY, Lu B, Yu C, Chen J, Ling QL, Cao J, Chi ZQ, Xu L, Liu JG. Liu Y, et al. J Neurosci. 2012 Aug 29;32(35):12005-17. doi: 10.1523/JNEUROSCI.0871-12.2012. J Neurosci. 2012. PMID: 22933785 Free PMC article. - Posttranslational regulation of AMPA receptor trafficking and function.
Lu W, Roche KW. Lu W, et al. Curr Opin Neurobiol. 2012 Jun;22(3):470-9. doi: 10.1016/j.conb.2011.09.008. Epub 2011 Oct 14. Curr Opin Neurobiol. 2012. PMID: 22000952 Free PMC article. Review. - Unlocking the secrets of the δ2 glutamate receptor: A gatekeeper for synaptic plasticity in the cerebellum.
Kohda K, Kakegawa W, Yuzaki M. Kohda K, et al. Commun Integr Biol. 2013 Nov 1;6(6):e26466. doi: 10.4161/cib.26466. Epub 2013 Sep 27. Commun Integr Biol. 2013. PMID: 24563706 Free PMC article.
References
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC (2000) Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci 3: 1291–1300 - PubMed
- Boxall AR, Lancaster B, Garthwaite J (1996) Tyrosine kinase is required for long-term depression in the cerebellum. Neuron 16: 805–813 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials