Nucleolar protein PinX1p regulates telomerase by sequestering its protein catalytic subunit in an inactive complex lacking telomerase RNA - PubMed (original) (raw)
. 2004 Feb 15;18(4):387-96.
doi: 10.1101/gad.1171804. Epub 2004 Feb 20.
Affiliations
- PMID: 14977919
- PMCID: PMC359393
- DOI: 10.1101/gad.1171804
Nucleolar protein PinX1p regulates telomerase by sequestering its protein catalytic subunit in an inactive complex lacking telomerase RNA
Jue Lin et al. Genes Dev. 2004.
Abstract
Human TRF1-binding protein PinX1 inhibits telomerase activity. Here we report that overexpression of yeast PinX1p (yPinX1p) results in shortened telomeres and decreased in vitro telomerase activity. yPinX1p coimmunoprecipitated with yeast telomerase protein Est2p even in cells lacking the telomerase RNA TLC1, or the telomerase-associated proteins Est1p and Est3p. Est2p regions required for binding to yPinX1p or TLC1 were similar. Furthermore, we found two distinct Est2p complexes exist, containing either yPinX1p or TLC1. Levels of Est2p-yPinX1p complex increased when TLC1 was deleted and decreased when TLC1 was overexpressed. Hence, we propose that yPinX1p regulates telomerase by sequestering its protein catalytic subunit in an inactive complex lacking telomerase RNA.
Figures
Figure 1.
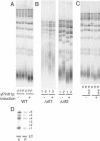
Overexpression of yPinX1p leads to shortened telomeres and decreased in vitro telomerase activity. (A) Southern blot of telomeres in wild-type cells (strain BY4705) overexpressing yPinX1p. (B) Δ_rif1_ (yEHB0234) and Δ_rif2_ (yEHB0235) strains overexpressing yPinX1p. (C) Wild-type cells overexpressing both yPinX1p and Est2p. (V) Vector plasmid; (P) yPinX1 overexpression plasmid with Gal promoter (pRS316GalPinX1); (E) Est2myc overexpression plasmid (pRS424Est2myc); (–) cells grown in glucose medium; (+) cells grown in galactose medium. In B, results from two independent transformants with pRS316GalPinX1 are shown. (D) In vitro telomerase activity assay. (LC) Loading control. A γ-32P-ATP-labeled oligonucleotide was added to the assay as the loading control. Products with 1–6 nt added to the primer are indicated.
Figure 2.
Coimmunoprecipitation of yPinX1p and Est2p. (A) Coimmunoprecipitation of Est2p-myc with yPinX1p-TAP using IgG beads. (B) Coimmunoprecipitation of yPinX1p-myc with Est2p-TAP using IgG beads. For Western blotting (WB), 2% of the extract used for coimmunoprecipitation experiments was loaded in whole-cell extract lanes.
Figure 3.
Coimmunoprecipitation of yPinX1p and Est2p is independent of TLC1 RNA, Est1p, or Est3p. (A) Overexpressed Est2p-myc copurified with yPinX1p-TAP in strain yEHB4084 containing wild-type TLC1, EST1, and EST3. Est2p-myc was on a 2μ plasmid (pRS424Est2-myc), and yPinX1-TAP was expressed from the Gal promoter on plasmid pRS316GalPinX1-TAP. (B) Est2p-myc copurified with yPinX1p in Δ_tlc1_, Δ_est1_, or Δ_est3_ strains. (S) S100; (B) IgG beads; (E) eluate from IgG beads. Fractions 1–5 are eluate from calmodulin beads. For Western blotting, 0.2% of total extract, 2% of IgG beads after binding, 2% of IgG beads eluate, and 20% of calmodulin eluate were loaded.
Figure 4.
The same region in Est2p is required for both yPinX1p and TLC1 binding. (A) Diagram of Est2p domain structures. (B) Est2p mutant proteins deleted of domain N, GQ, RT, or C copurify with yPinX1p. Est2p mutant proteins deleted of domain CP, QFP, or T do not copurify with yPinX1p. (S) S100; (B) IgG beads; (E) eluate from IgG beads. Fractions 1–5 are eluate from calmodulin beads. For Western blotting, 0.2% of total extract, 2% of IgG beads after binding, 2% of IgG beads eluate, and 20% of calmodulin eluate were loaded. (C) Est2p mutant proteins deleted of domain CP, QFP, or T do not bind TLC1. (Top panel) Western blotting of Est2p domain deletion mutants. (Lower panel) Northern blotting of TLC1 RNA. (WCE) RNA prepared from whole-cell extracts; (IP) RNA prepared from cell extracts immunoprecipitated with anti-myc antibody 9E10. For RNA preparation, 10% of input whole-cell extract was used and was loaded in WCE lanes.
Figure 5.
Detection of two distinct Est2p complexes: Est2p–TLC1 and Est2p–yPinX1p. (A) DEAE purified fractions contain TLC1 and Est2p, but no detectable yPinX1p. (S) S100 extract; (FT) flowthrough from DEAE column; (W) last wash from DEAE column; (#1–9) DEAE eluted fractions. (B) yPinX1p-TAP complex purified with IgG contained Est2p, but not TLC1. (S) S100 extract; (FT) flowthrough from IgG beads; (IP) immunoprecipitated samples. (Top panel) Northern blot analysis probed for TLC1 RNA. (Bottom panel) Western blot analysis probed for Est2p-myc. For Western blot analysis and preparation of RNA for Northern blot analysis, 10% of the input extract was used.
Figure 6.
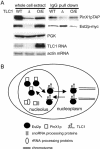
TLC1 levels influence yPinX1–Est2p binding efficiency. (A) Deletion of TLC1 results in more Est2p associated with yPinX1p, and overexpression of TLC1 results in less Est2p associated with yPinX1p. (O/E) TLC1 overexpressed from the GAL1 promoter. PGK serves as the loading control for Western blotting. The amount of TLC1 RNA is shown on the bottom with actin mRNA as the loading control. (B) Amodel for yPinX1p-mediated inhibition of telomerase activity. See text for description.
Similar articles
- Two pathways recruit telomerase to Saccharomyces cerevisiae telomeres.
Chan A, Boulé JB, Zakian VA. Chan A, et al. PLoS Genet. 2008 Oct;4(10):e1000236. doi: 10.1371/journal.pgen.1000236. Epub 2008 Oct 24. PLoS Genet. 2008. PMID: 18949040 Free PMC article. - Intracellular trafficking of yeast telomerase components.
Teixeira MT, Forstemann K, Gasser SM, Lingner J. Teixeira MT, et al. EMBO Rep. 2002 Jul;3(7):652-9. doi: 10.1093/embo-reports/kvf133. EMBO Rep. 2002. PMID: 12101098 Free PMC article. - The Est3 protein is a subunit of yeast telomerase.
Hughes TR, Evans SK, Weilbaecher RG, Lundblad V. Hughes TR, et al. Curr Biol. 2000 Jun 29;10(13):809-12. doi: 10.1016/s0960-9822(00)00562-5. Curr Biol. 2000. PMID: 10898986 - Telomerase: what are the Est proteins doing?
Taggart AK, Zakian VA. Taggart AK, et al. Curr Opin Cell Biol. 2003 Jun;15(3):275-80. doi: 10.1016/s0955-0674(03)00040-1. Curr Opin Cell Biol. 2003. PMID: 12787768 Review. - Maturation and shuttling of the yeast telomerase RNP: assembling something new using recycled parts.
Bartle L, Vasianovich Y, Wellinger RJ. Bartle L, et al. Curr Genet. 2022 Feb;68(1):3-14. doi: 10.1007/s00294-021-01210-2. Epub 2021 Sep 2. Curr Genet. 2022. PMID: 34476547 Free PMC article. Review.
Cited by
- The PinX1/NPM interaction associates with hTERT in early-S phase and facilitates telomerase activation.
Ho ST, Jin R, Cheung DH, Huang JJ, Shaw PC. Ho ST, et al. Cell Biosci. 2019 Jun 13;9:47. doi: 10.1186/s13578-019-0306-y. eCollection 2019. Cell Biosci. 2019. PMID: 31210926 Free PMC article. - Inhibition of DNA double-strand break repair by the Ku heterodimer in mrx mutants of Saccharomyces cerevisiae.
Wasko BM, Holland CL, Resnick MA, Lewis LK. Wasko BM, et al. DNA Repair (Amst). 2009 Feb 1;8(2):162-9. doi: 10.1016/j.dnarep.2008.09.010. Epub 2008 Nov 18. DNA Repair (Amst). 2009. PMID: 18992851 Free PMC article. - Increased Stability of Nucleolar PinX1 in the Presence of TERT.
Keo P, Choi JS, Bae J, Shim YH, Oh BK. Keo P, et al. Mol Cells. 2015 Sep;38(9):814-20. doi: 10.14348/molcells.2015.0144. Epub 2015 Jul 21. Mol Cells. 2015. PMID: 26194824 Free PMC article. - PinX1 is a novel microtubule-binding protein essential for accurate chromosome segregation.
Yuan K, Li N, Jiang K, Zhu T, Huo Y, Wang C, Lu J, Shaw A, Thomas K, Zhang J, Mann D, Liao J, Jin C, Yao X. Yuan K, et al. J Biol Chem. 2009 Aug 21;284(34):23072-82. doi: 10.1074/jbc.M109.001990. Epub 2009 Jun 24. J Biol Chem. 2009. PMID: 19553660 Free PMC article. - PinX1 is recruited to the mitotic chromosome periphery by Nucleolin and facilitates chromosome congression.
Li N, Yuan K, Yan F, Huo Y, Zhu T, Liu X, Guo Z, Yao X. Li N, et al. Biochem Biophys Res Commun. 2009 Jun 19;384(1):76-81. doi: 10.1016/j.bbrc.2009.04.077. Epub 2009 Apr 23. Biochem Biophys Res Commun. 2009. PMID: 19393617 Free PMC article.
References
- Arai K., Masutomi, K., Khurts, S., Kaneko, S., Kobayashi, K., and Murakami, S. 2002. Two independent regions of human telomerase reverse transcriptase are important for its oligomerization and telomerase activity. J. Biol. Chem. 277: 8538–8544. - PubMed
- Baldwin A.S. 1996. The NF-κB and IκB proteins: New discoveries and insights. Annu. Rev. Immunol. 14: 649–683. - PubMed
- Beattie T.L., Zhou, W., Robinson, M.O., and Harrington, L. 1998. Reconstitution of human telomerase activity in vitro. Curr. Biol. 8: 177–180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases