Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics - PubMed (original) (raw)
. 2004 Mar 2;101(9):3053-8.
doi: 10.1073/pnas.0400136101. Epub 2004 Feb 20.
Marc R Kok, Changyu Zheng, Ioannis Bossis, Jianghua Wang, Ana P Cotrim, Natanya Marracino, Corinne M Goldsmith, John A Chiorini, Y Peng Loh, Lynnette K Nieman, Bruce J Baum
Affiliations
- PMID: 14978265
- PMCID: PMC365743
- DOI: 10.1073/pnas.0400136101
Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics
Antonis Voutetakis et al. Proc Natl Acad Sci U S A. 2004.
Abstract
The use of critical-for-life organs (e.g., liver or lung) for systemic gene therapeutics can lead to serious safety concerns. To circumvent such issues, we have considered salivary glands (SGs) as an alternative gene therapeutics target tissue. Given the high secretory abilities of SGs, we hypothesized that administration of low doses of recombinant adeno-associated virus (AAV) vectors would allow for therapeutic levels of transgene-encoded secretory proteins in the bloodstream. We administered 10(9) particles of an AAV vector encoding human erythropoietin (hEPO) directly to individual mouse submandibular SGs. Serum hEPO reached maximum levels 8-12 weeks after gene delivery and remained relatively stable for 54 weeks (longest time studied). Hematocrit levels were similarly increased. Moreover, these effects proved to be vector dose-dependent, and even a dosage as low as 10(8) particles per animal led to significant increases in hEPO and hematocrit levels. Vector DNA was detected only within the targeted SGs, and levels of AAV copies within SGs were highly correlated with serum hEPO levels (r = 0.98). These results show that SGs appear to be promising targets with potential clinical applicability for systemic gene therapeutics.
Figures
Fig. 1.
AAVhEPO construct. The vector AAVhEPO was constructed as described in Materials and Methods. Inverted terminal repeat (ITR), Rous sarcoma virus promoter (RSV), hEPO cDNA, and polyadenylation signal (Poly-A) are shown. Arrows indicate restriction enzyme sites: _Sma_I (red arrows) and BssH II (blue arrows). Black line at top indicates the probe used in the Southern hybridization blot (see Fig. 7). Red and blue lines connecting arrows indicate the DNA fragments observed in the Southern hybridization blot after digestion with the specific enzymes.
Fig. 2.
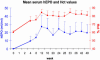
Serum hEPO and Hct levels in AAVhEPO-treated mice. Vector (AAVhEPO; 109 particles per animal) was administered to BALB/c mouse submandibular SGs (n = 5) by retrograde ductal delivery as described in Materials and Methods. Data shown are the mean (+SE) serum hEPO (blue line, left y axis), and Hct (red line, right y axis) levels measured over a 48-week period.
Fig. 3.
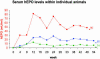
Serum hEPO levels in three individual mice after AAVhEPO administration. Vector (109 particles per animal) was delivered as in Fig. 2, and hEPO was measured over a 54-week period; longest time studied [*, animal died at 48 weeks, apparently of a stroke (hemiparesis observed shortly before death) or was killed at indicated time points]. Hct levels at the time of death or euthanasia for each individual mouse are shown after the last measurement point.
Fig. 4.
hEPO expression is viral vector dose-dependent. Three groups of BALB/c mice received 108, 109, or 5 × 109 particles per animal of the AAVhEPO vector via retrograde ductal cannulation of the submandibular SGs, as described in Fig. 2. Bars represent mean (+SE) serum hEPO levels 12 weeks after vector administration for each dosage group (n = 3, 5, and 4 animals per group, respectively).
Fig. 5.
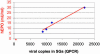
Relationship between hEPO expression in serum and viral copies present in SGs in treated and naïve animals. Mice were killed 8 weeks after administration (n = 4; 109 particles of AAVhEPO per treated animal or 50 μl 0.9% normal saline per naïve animal), and viral copies present in the SGs and serum hEPO levels were measured as described in Materials and Methods. The correlation between viral copies present and serum hEPO levels was highly significant (r = 0.98). The red asterisk indicates the mean hEPO serum levels and mean number of viral copies present in SGs of naïve animals (n = 4).
Fig. 6.
Immunocytochemical detection of β-galactosidase expression in mouse submandibular glands. Cryosections were prepared 8 weeks after AAVLacZ administration to mouse submandibular SGs (n = 5, 109 particles per animal), and β-galactosidase expression was detected by using an anti-β-galactosidase antibody. Sections were counterstained with hematoxylin. The β-galactosidase cDNA used contains a nuclear localization signal. Red arrows indicate representative cells with nuclear localized β-galactosidase. Staining was observed only in salivary ductal cells. No staining was detected in the control cryosections obtained from an animal receiving AAVhEPO by using the same immunocytochemistry procedure, as shown (Inset).
Fig. 7.
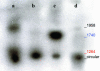
Southern hybridization blot of low-molecular-weight DNA from the SGs of AAVhEPO-treated animals. Vector (109 particles per animal) was administered, and Hirt-extracted DNA was prepared from SGs after 8 weeks, as described in Materials and Methods. Undigested Hirt-extracted DNA showed that the recombinant AAVhEPO viral DNA is 1,958 bp (lane a). Digestion of the Hirt-extracted DNA with _Sma_I (lane b) and _BssH_II (lane c) resulted in 1,264- and 1,740-bp fragments respectively, as expected (Fig. 1). Blue and red colors correspond to the red and blue lines in Fig. 1, indicating the restriction enzyme sites and the resulting DNA fragments. Digestion with Plasmid Safe (an enzyme only cutting linear DNA forms) indicated the presence of episomally maintained circular forms (lane d). Hirt-extracted DNA from naïve animals yielded no hybridization positive bands on Southern analyses (data not shown).
Similar articles
- Salivary glands as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders.
Voutetakis A, Bossis I, Kok MR, Zhang W, Wang J, Cotrim AP, Zheng C, Chiorini JA, Nieman LK, Baum BJ. Voutetakis A, et al. J Endocrinol. 2005 Jun;185(3):363-72. doi: 10.1677/joe.1.06171. J Endocrinol. 2005. PMID: 15930162 - Rapamycin control of transgene expression from a single AAV vector in mouse salivary glands.
Wang J, Voutetakis A, Papa M, Rivera VM, Clackson T, Lodde BM, Mineshiba F, Baum BJ. Wang J, et al. Gene Ther. 2006 Jan;13(2):187-90. doi: 10.1038/sj.gt.3302647. Gene Ther. 2006. PMID: 16177817 - Gender differences in serotype 2 adeno-associated virus biodistribution after administration to rodent salivary glands.
Voutetakis A, Zheng C, Wang J, Goldsmith CM, Afione S, Chiorini JA, Wenk ML, Vallant M, Irwin RD, Baum BJ. Voutetakis A, et al. Hum Gene Ther. 2007 Nov;18(11):1109-18. doi: 10.1089/hum.2007.072. Hum Gene Ther. 2007. PMID: 17939749 - Theodore E. Woodward Award. AAV-mediated gene transfer for hemophilia.
High KA. High KA. Trans Am Clin Climatol Assoc. 2003;114:337-51; discussion 351-2. Trans Am Clin Climatol Assoc. 2003. PMID: 12813929 Free PMC article. Review. - Utilizing endocrine secretory pathways in salivary glands for systemic gene therapeutics.
Voutetakis A, Wang J, Baum BJ. Voutetakis A, et al. J Cell Physiol. 2004 Apr;199(1):1-7. doi: 10.1002/jcp.10429. J Cell Physiol. 2004. PMID: 14978729 Review.
Cited by
- Effect of human vasoactive intestinal peptide gene transfer in a murine model of Sjogren's syndrome.
Lodde BM, Mineshiba F, Wang J, Cotrim AP, Afione S, Tak PP, Baum BJ. Lodde BM, et al. Ann Rheum Dis. 2006 Feb;65(2):195-200. doi: 10.1136/ard.2005.038232. Epub 2005 Jun 23. Ann Rheum Dis. 2006. PMID: 15975969 Free PMC article. - In vivo secretion of the mouse immunoglobulin G Fc fragment from rat submandibular glands.
Racz GZ, Perez-Riveros P, Adriaansen J, Zheng C, Baum BJ. Racz GZ, et al. J Gene Med. 2009 Jul;11(7):580-7. doi: 10.1002/jgm.1340. J Gene Med. 2009. PMID: 19424985 Free PMC article. - Including the p53 ELAV-like protein-binding site in vector cassettes enhances transgene expression in rat submandibular gland.
Zheng C, Baum BJ. Zheng C, et al. Oral Dis. 2012 Jul;18(5):477-84. doi: 10.1111/j.1601-0825.2011.01895.x. Epub 2012 Jan 18. Oral Dis. 2012. PMID: 22251132 Free PMC article. - HIF prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques.
Hsieh MM, Linde NS, Wynter A, Metzger M, Wong C, Langsetmo I, Lin A, Smith R, Rodgers GP, Donahue RE, Klaus SJ, Tisdale JF. Hsieh MM, et al. Blood. 2007 Sep 15;110(6):2140-7. doi: 10.1182/blood-2007-02-073254. Epub 2007 Jun 8. Blood. 2007. PMID: 17557894 Free PMC article. - Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity.
Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D, Chiorini JA. Schmidt M, et al. J Virol. 2008 Feb;82(3):1399-406. doi: 10.1128/JVI.02012-07. Epub 2007 Nov 28. J Virol. 2008. PMID: 18045941 Free PMC article.
References
- Felgner, P. L. & Rhodes, G. (1991) Nature 349, 351-352. - PubMed
- Crystal, R. G. (1995) Nat. Med. 1, 15-17. - PubMed
- Snyder, R. O., Miao, C. H., Patijn G. A., Spratt, S. K., Danos, O., Nagy, D., Gown, A. M., Winther, B., Meuse, L., Cohen, L. K., et al. (1997) Nat. Genet. 16, 270-276. - PubMed
- Johnston, J., Tazelaar, J., Rivera, V. M., Clackson, T., Gao, G. P. & Wilson, J. M. (2003) Mol. Ther. 7, 493-497. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical