Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of beta-catenin - PubMed (original) (raw)
Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of beta-catenin
Feng Cong et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Wnt signaling regulates many aspects of development by increasing the signaling activity of beta-catenin. Axin is a negative regulator of the Wnt signaling pathway, and it is responsible for the formation of the beta-catenin degradation complex. Genetic studies with Drosophila suggest that Axin promotes cytoplasmic localization of beta-catenin independent of Axin's known role of enhancing degradation of beta-catenin. Here, we show that Axin is a nuclear-cytoplasmic shuttling protein. Nuclear export of Axin depends on the chromosome maintenance region 1 nuclear receptor; treatment with the chromosome maintenance region 1 inhibitor leptomycin B induces nuclear accumulation of ectopically expressed or endogenous Axin. Functional nuclear localization and nuclear export signals have been mapped within Axin. Significantly, overexpression of an Axin fragment shifts coexpressed stabilized beta-catenin to the cytoplasm, and this effect requires shuttling of Axin between the cytoplasm and the nucleus. Our results suggest that Axin functions as a molecular chaperone for beta-catenin and that nuclear-cytoplasmic shuttling of Axin regulates the nuclear-cytoplasmic distribution of beta-catenin.
Figures
Fig. 1.
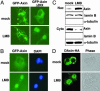
Nuclear-cytoplasmic shuttling of Axin. (A) 293 cells were transiently transfected with plasmids encoding GFP-Axin or GFP-AxinΔDIX. Thirty-six hours after transfection, cells were mock-treated or treated with 5 ng/ml LMB for 3 h and examined by fluorescence microscopy. (B) COS cells were infected with a retrovirus encoding GFP-Axin. Cells were mock-treated or treated with LMB for 3 h and examined by fluorescence microscopy. Nuclei were counterstained by 4′,6-diamidino-2-phenylindole. (C) 293 cells were mock-treated or treated with LMB for 3 h and subjected to subcellular fractionation. The levels of endogenous Axin in the nuclear and cytoplasmic fractions were determined by immunoblotting with anti-Axin antibodies. The relative purity of the nuclear and cytoplasmic fractions was confirmed by sequential probing for the nuclear marker lamin B and the cytoplasmic marker α-tubulin. (D) Drosophila S2 cells were transiently transfected with a plasmid expressing DAxin-HA. Cells were mock-treated or treated with LMB for 3 h, immunostained with anti-HA monoclonal antibodies and FITC-conjugated secondary antibodies, and examined by confocal microscopy. Cell shapes were examined by phase contrast microscopy.
Fig. 2.
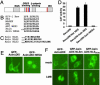
Characterization of NES and NLS sequences of Axin. (A) Schematic representation of the structure of Axin. Functional domains of Axin are labeled. The potential NES sequence is indicated by a blue bar, and proposed NLS sequences are indicated by red bars. (B) Alignment of selected NES sequences from known nuclear-cytoplasmic shuttling proteins with the Axin NES. The critical hydrophobic residues are shown in boldface. The last two conserved hydrophobic residues (Val-545 and Met-547) were substituted with Ala in the mutant form of Axin NES (Axin NESm). (C) Sequences of three potential NLSs of Axin. The basic residues in NLS1, NLS2, and bipartite NLS3 are shown in boldface. (D) Complementation of the nuclear export activity of Rev by the Axin NES. Rev mutants were coexpressed with pDM128 indicator construct and cytomegalovirus-Renilla control in 293 cells. Nuclear export and expression of unspliced CAT mRNA require the nuclear export activity of Rev. Controls include RevΔ3NI (NES-mutated Rev) and RevΔ3NI fused with the NES of human T-lymphotropic virus I Rex. The experiments were performed in triplicate, and CAT activities were normalized to Renilla luciferase activities. (E) Nuclear accumulation of NES-mutated AxinΔDIX. 293 cells were transiently transfected with plasmids expressing the wild-type or NES-mutated GFP-AxinΔDIX and examined by fluorescence microscopy. (F) Identification of NLS sequences of Axin. 293 cells were transiently transfected with plasmids encoding various forms of GFP-AxinΔDIX, mock-treated or treated with LMB for 3 h, and examined by fluorescence microscopy. Mutating three stretches of positively charged residues of GFP-AxinΔDIX abolished nuclear import of the protein upon LMB treatment. Nuclear import of this mutant protein was restored by adding to its C terminus a modified NLS from SV40 large T antigen.
Fig. 3.
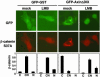
AxinΔDIX induces cytoplasmic shift of β-catenin in a CRM1-dependent manner. GFP-GST or GFP-AxinΔDIX was coexpressed with HA-tagged β-catenin S37A in 293 cells. Cells were treated with vehicle or LMB for 16 h. β-Catenin was detected by indirect immunofluorescence staining with anti-HA monoclonal antibodies and Texas red-conjugated secondary antibodies. The distribution of β-catenin in GFP-positive cells was scored as cytoplasmic (C), cytoplasmic and nuclear (CN), and nuclear (N) and graphed. The results are the average of three independent experiments; at least 300 GFP-positive cells were scored for each sample in every experiment.
Fig. 4.
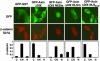
Nuclear-cytoplasmic shuttling is required for AxinΔDIX-induced cytoplasmic shift of β-catenin. GFP-GST or various forms of GFP-AxinΔDIX were coexpressed with HA-tagged β-catenin S37A in 293 cells. The distributions of HA-tagged β-catenin in GFP-positive cells were scored as in Fig. 3. The expression levels of various mutants of GFP-AxinΔDIX were similar as determined by immunoblotting with anti-GFP antibodies (data not shown).
Similar articles
- Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover.
Henderson BR. Henderson BR. Nat Cell Biol. 2000 Sep;2(9):653-60. doi: 10.1038/35023605. Nat Cell Biol. 2000. PMID: 10980707 - Nucleo-cytoplasmic shuttling of Axin, a negative regulator of the Wnt-beta-catenin Pathway.
Wiechens N, Heinle K, Englmeier L, Schohl A, Fagotto F. Wiechens N, et al. J Biol Chem. 2004 Feb 13;279(7):5263-7. doi: 10.1074/jbc.M307253200. Epub 2003 Nov 20. J Biol Chem. 2004. PMID: 14630927 - Cyclin-dependent kinase 2 regulates the interaction of Axin with beta-catenin.
Kim SI, Park CS, Lee MS, Kwon MS, Jho EH, Song WK. Kim SI, et al. Biochem Biophys Res Commun. 2004 Apr 30;317(2):478-83. doi: 10.1016/j.bbrc.2004.03.065. Biochem Biophys Res Commun. 2004. PMID: 15063782 - Regulation of beta-catenin signaling in the Wnt pathway.
Kikuchi A. Kikuchi A. Biochem Biophys Res Commun. 2000 Feb 16;268(2):243-8. doi: 10.1006/bbrc.1999.1860. Biochem Biophys Res Commun. 2000. PMID: 10679188 Review. - Axin: a master scaffold for multiple signaling pathways.
Luo W, Lin SC. Luo W, et al. Neurosignals. 2004 May-Jun;13(3):99-113. doi: 10.1159/000076563. Neurosignals. 2004. PMID: 15067197 Review.
Cited by
- Regulation of nuclear-cytoplasmic shuttling and function of Family with sequence similarity 13, member A (Fam13a), by B56-containing PP2As and Akt.
Jin Z, Chung JW, Mei W, Strack S, He C, Lau GW, Yang J. Jin Z, et al. Mol Biol Cell. 2015 Mar 15;26(6):1160-73. doi: 10.1091/mbc.E14-08-1276. Epub 2015 Jan 21. Mol Biol Cell. 2015. PMID: 25609086 Free PMC article. - Nuclear-cytoplasmic shuttling of menin regulates nuclear translocation of {beta}-catenin.
Cao Y, Liu R, Jiang X, Lu J, Jiang J, Zhang C, Li X, Ning G. Cao Y, et al. Mol Cell Biol. 2009 Oct;29(20):5477-87. doi: 10.1128/MCB.00335-09. Epub 2009 Aug 3. Mol Cell Biol. 2009. PMID: 19651895 Free PMC article. - Tankyrases: structure, function and therapeutic implications in cancer.
Haikarainen T, Krauss S, Lehtio L. Haikarainen T, et al. Curr Pharm Des. 2014;20(41):6472-88. doi: 10.2174/1381612820666140630101525. Curr Pharm Des. 2014. PMID: 24975604 Free PMC article. Review. - The Wnt/β-catenin pathway in human fibrotic-like diseases and its eligibility as a therapeutic target.
Enzo MV, Rastrelli M, Rossi CR, Hladnik U, Segat D. Enzo MV, et al. Mol Cell Ther. 2015 Jan 30;3:1. doi: 10.1186/s40591-015-0038-2. eCollection 2015. Mol Cell Ther. 2015. PMID: 26056602 Free PMC article. - Cytokinesis proteins Tum and Pav have a nuclear role in Wnt regulation.
Jones WM, Chao AT, Zavortink M, Saint R, Bejsovec A. Jones WM, et al. J Cell Sci. 2010 Jul 1;123(Pt 13):2179-89. doi: 10.1242/jcs.067868. Epub 2010 Jun 1. J Cell Sci. 2010. PMID: 20516152 Free PMC article.
References
- Cadigan, K. M. & Nusse, R. (1997) Genes Dev. 11, 3286-3305. - PubMed
- Wodarz, A. & Nusse, R. (1998) Annu. Rev. Cell Dev. Biol. 14, 59-88. - PubMed
- Polakis, P. (2000) Genes Dev. 14, 1837-1851. - PubMed
- Peifer, M. & Polakis, P. (2000) Science 287, 1606-1609. - PubMed
- Schneider, S., Steinbeisser, H., Warga, R. M. & Hausen, P. (1996) Mech. Dev. 57, 191-198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous