Telomere shortening occurs in subsets of normal breast epithelium as well as in situ and invasive carcinoma - PubMed (original) (raw)
Comparative Study
Telomere shortening occurs in subsets of normal breast epithelium as well as in situ and invasive carcinoma
Alan K Meeker et al. Am J Pathol. 2004 Mar.
Abstract
In the setting of inactivated DNA damage-sensitive checkpoints, critically shortened telomeres promote chromosomal instability and the types of widespread cytogenetic alterations that characterize most human carcinomas. Using a direct telomere fluorescence in situ hybridization technique, we analyzed 114 invasive breast carcinomas, 29 carcinoma in situ lesions, 10 benign proliferative lesions, and different normal epithelial components of the male and female breast. We found marked telomere shortening in the majority (52.5%) of invasive carcinomas; smaller subsets of invasive carcinoma demonstrated moderate telomere shortening (17.5%) or normal telomere lengths (21%), while a small subgroup (5%) contained elongated telomeres. Strikingly, the majority (78%) of ductal carcinoma in situ demonstrated markedly or moderately shortened telomeres. Surprisingly, unlike all other normal epithelia studied to date, moderate telomere shortening was observed in benign secretory cells in approximately 50% of histologically-normal terminal duct lobular units (from which most breast cancer is thought to arise), while such shortening was not seen in myoepithelial cells or normal large lactiferous ducts of the female breast or male breast ducts (from which breast cancer infrequently arises). We postulate that such shortening is the result of hormonally driven, physiological proliferation, and may delineate a population of epithelial cells at risk for subsequent malignant transformation.
Figures
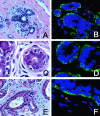
Figure 1
H&E staining (A, C, E) and TELI-FISH (B, D, F) analysis of telomere length in normal breast TDLU. A and B: Note that the secretory cells (negative with the green actin stain, facing the lumen) have comparable intensity of telomere signals as the ME cells (actin-positive). C and D: Note that the secretory cells in this TDLU have far less intense telomere signals that the ME cells. E and F: Note the variation in telomere signals among the secretory cells in this TDLU.
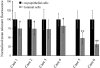
Figure 2
Quantitation of telomere lengths in normal TDLU. Mean DAPI-normalized telomere signal intensities were determined by digital image analysis for 10 to 20 randomly selected luminal and ME cells. Cases 1 to 4 were judged by visual inspection to have comparable telomere lengths between the two cell types, while telomeres appeared markedly shorter in the luminal cells of cases 5 and 6. (* P value <0.01, ** P value <0.00000001).
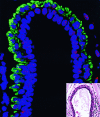
Figure 3
TELI-FISH analysis of telomere length in normal lactiferous duct. Note that the luminal cells (negative with the green actin stain, facing the lumen) have comparable intensity of telomere signals as the ME cells (actin-positive). Inset: H&E-stained adjacent section.
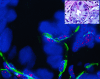
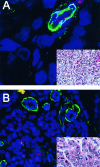
Figure 4
TELI-FISH analysis of telomere length in DCIS. Note that the luminal DCIS cells (negative with the green actin stain, facing the lumen) have virtually no telomere signal, in comparison to the ME cells (actin-positive). Inset: H&E-stained adjacent section.
Figure 5
TELI-FISH analysis of telomere length in invasive ductal carcinoma (IDC). Two examples of high-grade IDC are shown. A: This IDC has virtually undetectable telomere signals. Note the actin-positive pericytes of the capillary at the upper right of the figure, which, along with the benign endothelial cells, have intact telomere signals and hence serve as an internal control. B: This IDC has extremely bright telomere signals. Note the entrapped normal acini within the lesion, which are actin-positive. In this case, the tumor telomere signals are so strong that, at this setting, the normal acini and stromal telomere signals present are not visible in the image. Insets: adjacent H&E sections.
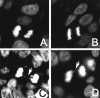
Figure 6
Anaphase bridges in DAPI-stained IDC and DCIS. A: Normal anaphase in an IDC with normal length telomeres. B: Normal anaphase in a DCIS with normal length telomeres. C: Anaphase bridge in an IDC with abnormally short telomeres. D: Anaphase bridge in a DCIS with abnormally short telomeres.
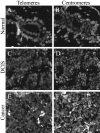
Figure 7
PNA probe accessibility controls. Serial tissue sections were hybridized with either telomere-specific PNA probe (A,C,E) or centromere-specific PNA probe (B,D,F). A and B: Normal breast TDLU. C and D: DCIS. E and F: Invasive carcinoma. Note the diminished telomere signals in these examples of normal luminal cells, DCIS and IDC, as compared to the robust centromere signals in these same cells.
Similar articles
- Elevated TRF2 in advanced breast cancers with short telomeres.
Diehl MC, Idowu MO, Kimmelshue KN, York TP, Jackson-Cook CK, Turner KC, Holt SE, Elmore LW. Diehl MC, et al. Breast Cancer Res Treat. 2011 Jun;127(3):623-30. doi: 10.1007/s10549-010-0988-7. Epub 2010 Jul 13. Breast Cancer Res Treat. 2011. PMID: 20625812 Free PMC article. - Telomere shortening occurs early during breast tumorigenesis: a cause of chromosome destabilization underlying malignant transformation?
Meeker AK, Argani P. Meeker AK, et al. J Mammary Gland Biol Neoplasia. 2004 Jul;9(3):285-96. doi: 10.1023/B:JOMG.0000048775.04140.92. J Mammary Gland Biol Neoplasia. 2004. PMID: 15557801 Review. - Luminal and cancer cells in the breast show more rapid telomere shortening than myoepithelial cells and fibroblasts.
Kurabayashi R, Takubo K, Aida J, Honma N, Poon SS, Kammori M, Izumiyama-Shimomura N, Nakamura K, Tsuji E, Matsuura M, Ogawa T, Kaminishi M. Kurabayashi R, et al. Hum Pathol. 2008 Nov;39(11):1647-55. doi: 10.1016/j.humpath.2008.04.005. Epub 2008 Jul 24. Hum Pathol. 2008. PMID: 18656239 - Hormones and progeny of breast tumor cells.
Schneider HP, Böcker W. Schneider HP, et al. Climacteric. 2006 Apr;9(2):88-107. doi: 10.1080/13697130600677435. Climacteric. 2006. PMID: 16698656 Review. - Telomere length on chromosome 17q shortens more than global telomere length in the development of breast cancer.
Rashid-Kolvear F, Pintilie M, Done SJ. Rashid-Kolvear F, et al. Neoplasia. 2007 Apr;9(4):265-70. doi: 10.1593/neo.07106. Neoplasia. 2007. PMID: 17460770 Free PMC article.
Cited by
- Generation of Immortalised But Unstable Cells after hTERT Introduction in Telomere-Compromised and p53-Deficient vHMECs.
Bernal A, Zafon E, Domínguez D, Bertran E, Tusell L. Bernal A, et al. Int J Mol Sci. 2018 Jul 17;19(7):2078. doi: 10.3390/ijms19072078. Int J Mol Sci. 2018. PMID: 30018248 Free PMC article. - Elevated TRF2 in advanced breast cancers with short telomeres.
Diehl MC, Idowu MO, Kimmelshue KN, York TP, Jackson-Cook CK, Turner KC, Holt SE, Elmore LW. Diehl MC, et al. Breast Cancer Res Treat. 2011 Jun;127(3):623-30. doi: 10.1007/s10549-010-0988-7. Epub 2010 Jul 13. Breast Cancer Res Treat. 2011. PMID: 20625812 Free PMC article. - Dynamic chromosomal rearrangements in Hodgkin's lymphoma are due to ongoing three-dimensional nuclear remodeling and breakage-bridge-fusion cycles.
Guffei A, Sarkar R, Klewes L, Righolt C, Knecht H, Mai S. Guffei A, et al. Haematologica. 2010 Dec;95(12):2038-46. doi: 10.3324/haematol.2010.030171. Epub 2010 Sep 7. Haematologica. 2010. PMID: 20823137 Free PMC article. - Classification of chromosome segregation errors in cancer.
Gisselsson D. Gisselsson D. Chromosoma. 2008 Dec;117(6):511-9. doi: 10.1007/s00412-008-0169-1. Epub 2008 Jun 6. Chromosoma. 2008. PMID: 18528701 Review. - T cell immune deficiency rather than chromosome instability predisposes patients with short telomere syndromes to squamous cancers.
Schratz KE, Flasch DA, Atik CC, Cosner ZL, Blackford AL, Yang W, Gable DL, Vellanki PJ, Xiang Z, Gaysinskaya V, Vonderheide RH, Rooper LM, Zhang J, Armanios M. Schratz KE, et al. Cancer Cell. 2023 Apr 10;41(4):807-817.e6. doi: 10.1016/j.ccell.2023.03.005. Epub 2023 Apr 2. Cancer Cell. 2023. PMID: 37037617 Free PMC article.
References
- Blasco MA. Telomerase beyond telomeres. Nat Rev Cancer. 2002;2:627–633. - PubMed
- Kim SH, Kaminker P, Campisi J. Telomeres, aging and cancer: in search of a happy ending. Oncogene. 2002;21:503–511. - PubMed
- Hackett JA, Greider CW. Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene. 2002;21:619–626. - PubMed
- Autexier C, Greider CW. Telomerase and cancer: revisiting the telomere hypothesis. Trends Biochem Sci. 1996;21:387–391. - PubMed
- Artandi SE, DePinho RA. A critical role for telomeres in suppressing and facilitating carcinogenesis. Curr Opin Genet Dev. 2000;10:39–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA088843/CA/NCI NIH HHS/United States
- P50 CA058236/CA/NCI NIH HHS/United States
- T32DK07552/DK/NIDDK NIH HHS/United States
- CA58236/CA/NCI NIH HHS/United States
- CA88843/CA/NCI NIH HHS/United States
- T32 DK007552/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical