Expression of CCL28 by Reed-Sternberg cells defines a major subtype of classical Hodgkin's disease with frequent infiltration of eosinophils and/or plasma cells - PubMed (original) (raw)
Expression of CCL28 by Reed-Sternberg cells defines a major subtype of classical Hodgkin's disease with frequent infiltration of eosinophils and/or plasma cells
Hitoshi Hanamoto et al. Am J Pathol. 2004 Mar.
Abstract
Classical Hodgkin's disease (HD) is characterized by rare neoplastic Hodgkin and Reed-Sternberg (H-RS) cells within abundant reactive cellular backgrounds. In most cases, H-RS cells originate from the B-cell lineage, but their immunophenotypes are unusual. Here we newly found frequent expression of chemokine receptors CXCR6 and CCR10 and their respective ligands CXCL16 and CCL28 in HD-derived cell lines. CCR10 is known to be selectively expressed by plasma cells, whereas CCL28 attracts eosinophils via CCR3 and plasma cells via CCR10 and CCR3. Therefore, we examined their expression in HD tissues by immunohistochemistry. We found that H-RS cells in 15 of 19 cases were positive for CCL28. Among them, seven cases were also positive for CCR10, suggesting a potential autocrine effect. In situ hybridization confirmed the expression of CCL28 mRNA in H-RS cells. The CCL28 positivity in H-RS cells did not significantly correlate with that of LMP-1, CCL17, CCL22, or CCL11. However, it significantly correlated with the background accumulation of eosinophils, plasma cells, and CCR10+ cells. Thus, the production of CCL28 by H-RS cells may play a major role in tissue accumulation of eosinophils and/or plasma cells in classical HD. The frequent expression of CCR10 in H-RS cells themselves also supports their close relationship to plasma cells.
Figures
Figure 1
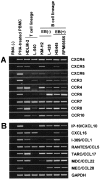
RT-PCR analysis on expression of chemokine receptors and chemokines in HD-derived cell lines. Total RNA was prepared from PBMCs stimulated with phytohemagglutinin for 3 days (positive control) and six HD-derived cell lines. RT-PCR was performed to examine the expression of 18 chemokine receptors (A) and 21 chemokines (B) (see Table 1 for primers). For details, see Materials and Methods. This figure shows the highlights of the representative results from at least two experiments.
Figure 2
Flow cytometric analysis on surface expression of chemokine receptors on HD-derived cell lines. Six HD-derived cell lines were stained for indicated chemokine receptors by using specific antibodies or CCL27-Fc chimera protein. Isotype-matched IgG or Fc protein served as negative controls. For details, see Materials and Methods. Representative results from at least two separate experiments are shown. Percentages of positive cells are indicated.
Figure 3
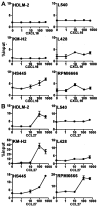
Chemotactic response of HD-derived cell lines to CXCL16 and CCL27. Indicated HD-derived cell lines were examined for chemotactic responses to CXCL16, the ligand for CXCR6 (A), or to CCL27, the monospecific ligand for CCR10 (B), both at nM. For details, see Materials and Methods. Representative results from at least two separate assays are shown.
Figure 4
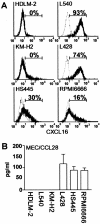
Surface expression of CXCL16 and secretion of CCL28 by HD-derived cell lines. A: Six HD-derived cell lines were stained for surface CXCL16 by using goat anti-CXCL16. Isotype-matched goat IgG served as negative control. For details, see Materials and Methods. Representative results from at least two separate experiments are shown. Percentages of positive cells are indicated. B: Cells from indicated HD-derived cell lines were seeded in a 24-well plate at a density of 1 × 106 cells/well and cultured for 3 days. CCL28 secreted in culture supernatants was measured with enzyme-linked immunosorbent assay. All assays were done in triplicate. For details, see Materials and Methods. Representative results from two separate experiments are shown.
Figure 5
Immunohistochemical analysis of HD tissues. Classical HD tissue specimens were examined with the following reagents: A, anti-LMP-1 (case 6); B, anti-TARC/CCL17 (case 6); C, anti-MDC/CCL22 (case 6); D, anti-CCR4 (case 13); E, anti-MEC/CCL28 (case 7); F, anti-MEC/CCL28 (case 5); G, anti-CCR10 (case 7); H, anti-CCR10 (case 6); I, anti-eotaxin/CCL11 (case 13); J, a representative isotype control (case 5); K, H&E staining (case 5); L, anti-CD138/syndecan-1 (case 6). For details, see Materials and Methods. All experiments were done at least twice and representative results are shown. Asterisks indicate H-RS cells. Original magnifications, ×1000.
Figure 6
In situ hybridization for MEC/CCL28. Expression of CCL28 mRNA in HD tissues was examined by in situ hybridization using DAKO’s Gene Point System (DAKO Japan). For details, see Materials and Methods. Representative results from at least two separate experiments are shown. A: Hybridization with anti-sense (case 7). B: Hybridization with sense probe (case 7), hybridization with anti-sense probe (case 4), hybridization with sense probe (case 4). Asterisks indicate H-RS cells. Original magnifications, ×1000.
Similar articles
- Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues.
Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. Nakayama T, et al. J Immunol. 2003 Feb 1;170(3):1136-40. doi: 10.4049/jimmunol.170.3.1136. J Immunol. 2003. PMID: 12538668 - High expression of the CC chemokine TARC in Reed-Sternberg cells. A possible explanation for the characteristic T-cell infiltratein Hodgkin's lymphoma.
van den Berg A, Visser L, Poppema S. van den Berg A, et al. Am J Pathol. 1999 Jun;154(6):1685-91. doi: 10.1016/S0002-9440(10)65424-7. Am J Pathol. 1999. PMID: 10362793 Free PMC article. - Infiltration of Th1 and Th2 lymphocytes around Hodgkin and Reed-Sternberg (H&RS) cells in Hodgkin disease: Relation with expression of CXC and CC chemokines on H&RS cells.
Ohshima K, Tutiya T, Yamaguchi T, Suzuki K, Suzumiya J, Kawasaki C, Haraoka S, Kikuchi M. Ohshima K, et al. Int J Cancer. 2002 Apr 1;98(4):567-72. doi: 10.1002/ijc.10218. Int J Cancer. 2002. PMID: 11920617 - The role of interleukin-3 in classical Hodgkin's disease.
Aldinucci D, Olivo K, Lorenzon D, Poletto D, Gloghini A, Carbone A, Pinto A. Aldinucci D, et al. Leuk Lymphoma. 2005 Mar;46(3):303-11. doi: 10.1080/10428190400013712. Leuk Lymphoma. 2005. PMID: 15621820 Review. - Chemokines, cytokines and their receptors in Hodgkin's lymphoma cell lines and tissues.
Maggio E, van den Berg A, Diepstra A, Kluiver J, Visser L, Poppema S. Maggio E, et al. Ann Oncol. 2002;13 Suppl 1:52-6. doi: 10.1093/annonc/13.s1.52. Ann Oncol. 2002. PMID: 12078904 Review.
Cited by
- Plasma cells in classical Hodgkin lymphoma: a new player in the microenvironment?
Visser L. Visser L. Br J Haematol. 2019 Jan;184(2):119-120. doi: 10.1111/bjh.15704. Epub 2018 Nov 28. Br J Haematol. 2019. PMID: 30485402 Free PMC article. No abstract available. - Immune Biomarkers in the Peripheral Blood and Tumor Microenvironment of Classical Hodgkin Lymphoma Patients in Relation to Tumor Burden and Response to Treatment.
Mulder TA, Andersson ML, Peña-Pérez L, Heimersson K, Xagoraris I, Wahlin BE, Månsson R, Hansson L, Rassidakis G, Palma M. Mulder TA, et al. Hemasphere. 2022 Oct 26;6(11):e794. doi: 10.1097/HS9.0000000000000794. eCollection 2022 Nov. Hemasphere. 2022. PMID: 36325271 Free PMC article. - Tumor-Infiltrating Macrophages in Post-Transplant, Relapsed Classical Hodgkin Lymphoma Are Donor-Derived.
Crane GM, Samols MA, Morsberger LA, Yonescu R, Thiess ML, Batista DA, Ning Y, Burns KH, Vuica-Ross M, Borowitz MJ, Gocke CD, Ambinder RF, Duffield AS. Crane GM, et al. PLoS One. 2016 Sep 29;11(9):e0163559. doi: 10.1371/journal.pone.0163559. eCollection 2016. PLoS One. 2016. PMID: 27685855 Free PMC article. - CXCR6 induces prostate cancer progression by the AKT/mammalian target of rapamycin signaling pathway.
Wang J, Lu Y, Wang J, Koch AE, Zhang J, Taichman RS. Wang J, et al. Cancer Res. 2008 Dec 15;68(24):10367-76. doi: 10.1158/0008-5472.CAN-08-2780. Cancer Res. 2008. PMID: 19074906 Free PMC article. Retracted. - Effect of inflammatory cytokines and plasma metabolome on OSA: a bidirectional two- sample Mendelian randomization study and mediation analysis.
Sun X, Wang C, He Y, Chen K, Miao Y. Sun X, et al. Front Immunol. 2024 Sep 16;15:1416870. doi: 10.3389/fimmu.2024.1416870. eCollection 2024. Front Immunol. 2024. PMID: 39351220 Free PMC article.
References
- Weiss LM, Chan JKC, MacLennan K, Warnke RA. Pathology of classical Hodgkin’s disease. Mauch PM, Armitage JO, Diehl V, Hoppe RT, Weiss LM, editors. Philadelphia: Lippincott, Williams, and Wilkins,; Hodgkin’s Disease. 1999:pp 101–120.
- Marafioti T, Hummel M, Anagnostopoulos I, Foss HD, Falini B, Delsol G, Isaacson PG, Pileri S, Stein H. Origin of nodular lymphocyte-predominant Hodgkin’s disease from a clonal expansion of highly mutated germinal-center B cells. N Engl J Med. 1997;337:453–458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical